First-line treatment with bortezomib rapidly stimulates both osteoblast activity and bone matrix deposition in patients with multiple myeloma, and stimulates osteoblast proliferation and differentiation in vitro
- PMID: 20528908
- PMCID: PMC2970902
- DOI: 10.1111/j.1600-0609.2010.01485.x
First-line treatment with bortezomib rapidly stimulates both osteoblast activity and bone matrix deposition in patients with multiple myeloma, and stimulates osteoblast proliferation and differentiation in vitro
Abstract
Objectives: The aim of the study was to investigate the effect of bortezomib on osteoblast proliferation and differentiation, as well as on bone matrix deposition for the first time in bisphosphonate-naïve, previously untreated patients with myeloma.
Methods: Twenty newly diagnosed patients received four cycles of bortezomib treatment, initially as monotherapy and then combined with a glucocorticoid from cycle two to four. Bone remodeling markers were monitored closely during treatment. Furthermore, the effects of bortezomib and a glucocorticoid on immature and mature osteoblasts were also studied in vitro.
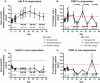
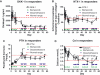
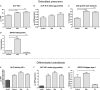
Results: Treatment with bortezomib caused a significant increase in bone-specific alkaline phosphatase and pro-collagen type I N-terminal propeptide, a novel bone formation marker. The addition of a glucocorticoid resulted in a transient decrease in collagen deposition. In vitro bortezomib induced osteoblast proliferation and differentiation. Differentiation but not proliferation was inhibited by glucocorticoid treatment.
Conclusions: Bortezomib used as first-line treatment significantly increased collagen deposition in patients with multiple myeloma and osteolytic lesions, but the addition of a glucocorticoid to the treatment transiently inhibited the positive effect of bortezomib, suggesting that bortezomib may result in better healing of osteolytic lesions when used without glucocorticoids in patients that have obtained remission with a previous therapy. The potential bone-healing properties of single-agent bortezomib are currently being explored in a clinical study in patients who have undergone high-dose therapy and autologous stem cell transplantation.
© 2010 John Wiley & Sons A/S.
Figures

References
-
- Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21–33. - PubMed
-
- Farrugia AN, Atkins GJ, To LB, Pan B, Horvath N, Kostakis P, et al. Receptor activator of nuclear factor-kappaB ligand expression by human myeloma cells mediates osteoclast formation in vitro and correlates with bone destruction in vivo. Cancer Res. 2003;63:5438–45. - PubMed
-
- Giuliani N, Bataille R, Mancini C, Lazzaretti M, Barille S. Myeloma cells induce imbalance in the osteoprotegerin/osteoprotegerin ligand system in the human bone marrow environment. Blood. 2001;98:3527–33. - PubMed
-
- Michigami T, Shimizu N, Williams PJ, Niewolna M, Dallas SL, Mundy GR, et al. Cell-cell contact between marrow stromal cells and myeloma cells via VCAM-1 and alpha(4)beta(1)-integrin enhances production of osteoclast-stimulating activity. Blood. 2000;96:1953–60. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

