Detection and prevention of ocular phototoxicity of ciprofloxacin and other fluoroquinolone antibiotics
- PMID: 20528972
- PMCID: PMC2910230
- DOI: 10.1111/j.1751-1097.2010.00755.x
Detection and prevention of ocular phototoxicity of ciprofloxacin and other fluoroquinolone antibiotics
Abstract
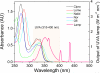
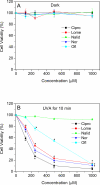
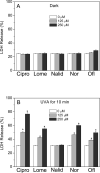
Fluoroquinolone (FLQ) drugs are a potent family of antibiotics used to treat infections including ocular infections. To determine if these antibiotics may be phototoxic to the eye, we exposed human lens epithelial cells to 0.125-1 mm FLQs (ciprofloxacin [Cipro], lomefloxacin [Lome], norfloxacin [Nor] and ofloxacin [Ofl]), the precursor quinolone nalidixic acid (Nalid) and UVA radiation (2.5 J cm(-2)). Based on fluorescence confocal microscopy, FLQs are diffused throughout the cytoplasm and preferentially located in the lysosomes of lens epithelial cells. Neither FLQ exposure alone nor UVA exposure alone reduced cell viability. However, with exposure to UVA radiation the FLQs studied (Cipro, Nor, Lome and Ofl) induced a phototoxic reaction that included necrosis, apoptosis, loss of cell viability as measured by MTS, and membrane damage as determined by the lactate dehydrogenase assay. Both Nalid and all FLQs studied (Cipro, Nor, Lome and Ofl) photopolymerized the lens protein alpha-crystallin. Phototoxic damage to lens epithelial cells and/or alpha-crystallin will lead to a loss of transparency of the human lens. However, if precautions are taken to filter all UV radiation from the eye while taking these antibiotics, eye damage may be prevented.
Figures




Similar articles
-
Phototoxicity in human lens epithelial cells promoted by St. John's Wort.Photochem Photobiol. 2004 Nov-Dec;80(3):583-6. doi: 10.1562/0031-8655(2004)080<0583:PIHLEC>2.0.CO;2. Photochem Photobiol. 2004. PMID: 15623347
-
Phototoxicity and cytotoxicity of fullerol in human lens epithelial cells.Toxicol Appl Pharmacol. 2008 Apr 1;228(1):49-58. doi: 10.1016/j.taap.2007.12.010. Epub 2007 Dec 15. Toxicol Appl Pharmacol. 2008. PMID: 18234258 Free PMC article.
-
Difference in phototoxicity of cyclodextrin complexed fullerene [(gamma-CyD)2/C60] and its aggregated derivatives toward human lens epithelial cells.Chem Res Toxicol. 2009 Apr;22(4):660-7. doi: 10.1021/tx800478u. Chem Res Toxicol. 2009. PMID: 19281132 Free PMC article.
-
Damage to mitochondria of cultured human skin fibroblasts photosensitized by fluoroquinolones.J Photochem Photobiol B. 2000 Oct;58(1):20-5. doi: 10.1016/s1011-1344(00)00101-9. J Photochem Photobiol B. 2000. PMID: 11195848
-
Fluoroquinolones: Blessings Or Curses.Curr Drug Targets. 2020;21(13):1354-1370. doi: 10.2174/1389450121666200621193355. Curr Drug Targets. 2020. PMID: 32564750 Review.
Cited by
-
Do fluoroquinolones increase aortic aneurysm or dissection incidence and mortality? A systematic review and meta-analysis.Front Cardiovasc Med. 2022 Aug 9;9:949538. doi: 10.3389/fcvm.2022.949538. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36017083 Free PMC article.
-
Phototoxicity of nano titanium dioxides in HaCaT keratinocytes--generation of reactive oxygen species and cell damage.Toxicol Appl Pharmacol. 2012 Aug 15;263(1):81-8. doi: 10.1016/j.taap.2012.06.001. Epub 2012 Jun 13. Toxicol Appl Pharmacol. 2012. PMID: 22705594 Free PMC article.
-
Adverse effects of antimicrobials via predictable or idiosyncratic inhibition of host mitochondrial components.Antimicrob Agents Chemother. 2012 Aug;56(8):4046-51. doi: 10.1128/AAC.00678-12. Epub 2012 May 21. Antimicrob Agents Chemother. 2012. PMID: 22615289 Free PMC article. Review.
-
The effects of exposure to solar radiation on human health.Photochem Photobiol Sci. 2023 May;22(5):1011-1047. doi: 10.1007/s43630-023-00375-8. Epub 2023 Mar 1. Photochem Photobiol Sci. 2023. PMID: 36856971 Free PMC article.
-
Current progress of fluoroquinolones-increased risk of aortic aneurysm and dissection.BMC Cardiovasc Disord. 2021 Sep 28;21(1):470. doi: 10.1186/s12872-021-02258-1. BMC Cardiovasc Disord. 2021. PMID: 34583637 Free PMC article. Review.
References
-
- Domagala JM. Structure-activity and structure-side-effect relationships for the quinolone antibacterials. J. Antimicrob. Chemother. 1994;33:685–706. - PubMed
-
- Goldstein EJ, Citron DM, Bendon L, Vagvolgyi AE, Trousdale MD, Appleman MD. Potential of topical norfloxacin therapy. Comparative in vitro activity against clinical ocular bacterial isolates. Arch. Ophthalmol. 1987;105:991–994. - PubMed
-
- Hobden JA, Reidy JJ, O'Callaghan RJ, Insler MS, Hill JM. Quinolones in collagen shields to treat aminoglycoside-resistant pseudomonal keratitis. Invest. Ophthalmol. Vis. Sci. 1990;31:2241–2243. - PubMed
-
- Malet F, Colin J, Jauch A, Abalain ML. Bacterial keratitis therapy in guinea pigs with lomefloxacin by initially high-followed by low-dosage regimen. Ophthalmic. Res. 1995;27:322–329. - PubMed
-
- Osato MS, Jensen HG, Trousdale MD, Bosso JA, Borrmann LR, Frank J, Akers P. The comparative in vitro activity of ofloxacin and selected ophthalmic antimicrobial agents against ocular bacterial isolates. Am. J. Ophthalmol. 1989;108:380–386. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

