Distinct synaptic properties of perisomatic inhibitory cell types and their different modulation by cholinergic receptor activation in the CA3 region of the mouse hippocampus
- PMID: 20529124
- PMCID: PMC2916217
- DOI: 10.1111/j.1460-9568.2010.07292.x
Distinct synaptic properties of perisomatic inhibitory cell types and their different modulation by cholinergic receptor activation in the CA3 region of the mouse hippocampus
Abstract
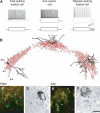
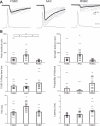
Perisomatic inhibition originates from three types of GABAergic interneurons in cortical structures, including parvalbumin-containing fast-spiking basket cells (FSBCs) and axo-axonic cells (AACs), as well as cholecystokinin-expressing regular-spiking basket cells (RSBCs). These interneurons may have significant impact in various cognitive processes, and are subjects of cholinergic modulation. However, it is largely unknown how cholinergic receptor activation modulates the function of perisomatic inhibitory cells. Therefore, we performed paired recordings from anatomically identified perisomatic interneurons and pyramidal cells in the CA3 region of the mouse hippocampus. We determined the basic properties of unitary inhibitory postsynaptic currents (uIPSCs) and found that they differed among cell types, e.g. GABA released from axon endings of AACs evoked uIPSCs with the largest amplitude and with the longest decay measured at room temperature. RSBCs could also release GABA asynchronously, the magnitude of the release increasing with the discharge frequency of the presynaptic interneuron. Cholinergic receptor activation by carbachol significantly decreased the uIPSC amplitude in all three types of cell pairs, but to different extents. M2-type muscarinic receptors were responsible for the reduction in uIPSC amplitudes in FSBC- and AAC-pyramidal cell pairs, while an antagonist of CB(1) cannabinoid receptors recovered the suppression in RSBC-pyramidal cell pairs. In addition, carbachol suppressed or even eliminated the short-term depression of uIPSCs in FSBC- and AAC-pyramidal cell pairs in a frequency-dependent manner. These findings suggest that not only are the basic synaptic properties of perisomatic inhibitory cells distinct, but acetylcholine can differentially control the impact of perisomatic inhibition from different sources.
Figures


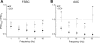


References
-
- Aronstam RS, Narayanan TK. Temperature effect on the detection of muscarinic receptor-G protein interactions in ligand binding assays. Biochem. Pharmacol. 1988;37:1045–1049. - PubMed
-
- Behrends JC, ten Bruggencate G. Cholinergic modulation of synaptic inhibition in the guinea pig hippocampus in vitro: excitation of GABAergic interneurons and inhibition of GABA-release. J. Neurophysiol. 1993;69:626–629. - PubMed
-
- Binda F, Bossi E, Giovannardi S, Forlani G, Peres A. Temperature effects on the presteady-state and transport-associated currents of GABA cotransporter rGAT1. FEBS Lett. 2002;512:303–307. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

