Degradation of human collagen isoforms by Clostridium collagenase and the effects of degradation products on cell migration
- PMID: 20529148
- PMCID: PMC7951583
- DOI: 10.1111/j.1742-481X.2010.00659.x
Degradation of human collagen isoforms by Clostridium collagenase and the effects of degradation products on cell migration
Abstract
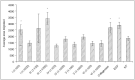
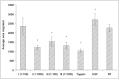
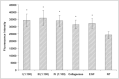
Clostridium collagenase has been widely used in biomedical research to dissociate tissues and isolate cells; and, since 1965, as a therapeutic drug for the removal of necrotic wound tissues. Previous studies found that purified collagenase-treated extracellular matrix stimulated cellular response to injury and increased cell proliferation and migration. This article presents an in vitro study investigating the digestive ability of Clostridium collagenase on human collagen types I, III, IV, V and VI. Our results showed that Clostridium collagenase displays proteolytic power to digest all these types of human collagen, except type VI. The degradation products derived were tested in cell migration assays using human keratinocytes (gold surface migration assay) and fibroblasts (chemotaxis cell migration assay). Clostridium collagenase itself and the degradation products of type I and III collagens showed an increase in keratinocyte and fibroblast migration, type IV-induced fibroblast migration only, and the remainder showed no effects compared with the control. The data indicate that Clostridium collagenase can effectively digest collagen isoforms that are present in necrotic wound tissues and suggest that collagenase treatment provides several mechanisms to enhance cell migration: collagenase itself and collagen degradation products.
Figures

Similar articles
-
Directed migration of murine and human tumor cells to collagenases and other proteases.Cancer Res. 1989 Sep 1;49(17):4835-41. Cancer Res. 1989. PMID: 2547519
-
The activity of collagenase-1 is required for keratinocyte migration on a type I collagen matrix.J Cell Biol. 1997 Jun 16;137(6):1445-57. doi: 10.1083/jcb.137.6.1445. J Cell Biol. 1997. PMID: 9182674 Free PMC article.
-
Loss of stiffness in collagen-rich uterine fibroids after digestion with purified collagenase Clostridium histolyticum.Am J Obstet Gynecol. 2016 Nov;215(5):596.e1-596.e8. doi: 10.1016/j.ajog.2016.05.006. Epub 2016 May 10. Am J Obstet Gynecol. 2016. PMID: 27177523
-
Collagen V Is a Potential Substrate for Clostridial Collagenase G in Pancreatic Islet Isolation.J Diabetes Res. 2016;2016:4396756. doi: 10.1155/2016/4396756. Epub 2016 Apr 18. J Diabetes Res. 2016. PMID: 27195301 Free PMC article.
-
Collagenases and collagen degradation.J Invest Dermatol. 1982 Jul;79 Suppl 1:83s-86s. doi: 10.1111/1523-1747.ep12545849. J Invest Dermatol. 1982. PMID: 6282982 Review.
Cited by
-
Programmed biomolecule delivery to enable and direct cell migration for connective tissue repair.Nat Commun. 2017 Nov 24;8(1):1780. doi: 10.1038/s41467-017-01955-w. Nat Commun. 2017. PMID: 29176654 Free PMC article.
-
Radiopaque Hemocompatible Ruminant-Sourced Gut Material with Antimicrobial Physiognomies for Biomedical Applications in Diabetics.ACS Omega. 2017 Mar 31;2(3):755-764. doi: 10.1021/acsomega.6b00373. Epub 2017 Mar 2. ACS Omega. 2017. PMID: 30023615 Free PMC article.
-
Maturation State and Matrix Microstructure Regulate Interstitial Cell Migration in Dense Connective Tissues.Sci Rep. 2018 Feb 19;8(1):3295. doi: 10.1038/s41598-018-21212-4. Sci Rep. 2018. PMID: 29459687 Free PMC article.
-
Repair of dense connective tissues via biomaterial-mediated matrix reprogramming of the wound interface.Biomaterials. 2015 Jan;39:85-94. doi: 10.1016/j.biomaterials.2014.10.067. Epub 2014 Nov 15. Biomaterials. 2015. PMID: 25477175 Free PMC article.
-
Factors Affecting Cell Viability during the Enzymatic Dissociation of Human Endocrine Tumor Tissues.Biology (Basel). 2024 Aug 27;13(9):665. doi: 10.3390/biology13090665. Biology (Basel). 2024. PMID: 39336093 Free PMC article.
References
-
- French MF, Bhown A, Van Wart HE. Limited proteolysis of types I, II and III collagens at hyper‐reactive sites by Clostridium histolyticum collagenase. Matrix Suppl 1992;1:134–5. - PubMed
-
- Mallya SK, Mookhtiar KA, Van Wart HE. Accurate, quantitative assays for the hydrolysis of soluble type I, II, and III3H‐acetylated collagens by bacterial and tissue collagenases. Anal Biochem 1986;158:334–45. - PubMed
-
- Grant NH, Alburn HE. Studies on the collagenases of Clostridium histolyticum. Arch Biochem Biophys 1959;82:245–55. - PubMed
-
- Mandl I, Keller S, Manahan J. Multiplicity of Clostridium histolyticum collagenases. Biochemistry 1964;3:1737–41. - PubMed
-
- Kono T. Purification and partial characterization of collagenolytic enzymes from Clostridium histolyticum. Biochemistry 1968;7:1106–14. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

