Transcellular transport of West Nile virus-like particles across human endothelial cells depends on residues 156 and 159 of envelope protein
- PMID: 20529314
- PMCID: PMC2889955
- DOI: 10.1186/1471-2180-10-165
Transcellular transport of West Nile virus-like particles across human endothelial cells depends on residues 156 and 159 of envelope protein
Abstract
Background: West Nile virus (WNV) causes viremia after invasion to the hosts by mosquito bite. Endothelial cells could play an important role in WNV spread from the blood stream into the central nervous system and peripheral tissues. Here, we analyzed the capacity of virus-like particles (VLPs) of the highly virulent NY99 6-LP strain (6-LP VLPs) and the low virulence Eg101 strain (Eg VLPs) to cross cultured human endothelial cells.
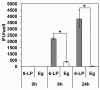
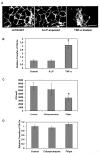
Results: 6-LP VLPs were transported from the apical to basolateral side of endothelial cells, whereas Eg VLPs were hardly transported. The localization of tight junction marker ZO-1 and the integrity of tight junctions were not impaired during the transport of 6-LP VLPs. The transport of 6-LP VLPs was inhibited by treatment with filipin, which prevents the formation of cholesterol-dependent membrane rafts, suggesting the involvement of raft-associated membrane transport. To determine the amino acid residues responsible for the transport of VLPs, we produced mutant VLPs, in which residues of E protein were exchanged between the 6-LP and Eg strains. Double amino acid substitution of the residues 156 and 159 greatly impaired the transport of VLPs.
Conclusion: Our results suggest that a transcellular pathway is associated with 6-LP VLPs transport. We also showed that the combination of the residues 156 and 159 plays an important role in the transport of VLPs across endothelial cells.
Figures

Similar articles
-
Identification of residues in West Nile virus pre-membrane protein that influence viral particle secretion and virulence.J Gen Virol. 2012 Sep;93(Pt 9):1965-1975. doi: 10.1099/vir.0.044453-0. Epub 2012 Jul 4. J Gen Virol. 2012. PMID: 22764317
-
Amino Acids at Positions 156 and 332 in the E Protein of the West Nile Virus Subtype Kunjin Virus Classical Strain OR393 Are Involved in Plaque Size, Growth, and Pathogenicity in Mice.Viruses. 2024 Aug 1;16(8):1237. doi: 10.3390/v16081237. Viruses. 2024. PMID: 39205211 Free PMC article.
-
Effects of the number of amino acid residues in the signal segment upstream or downstream of the NS2B-3 cleavage site on production and secretion of prM/M-E virus-like particles of West Nile virus.Microbes Infect. 2009 Nov;11(13):1019-28. doi: 10.1016/j.micinf.2009.07.009. Epub 2009 Aug 7. Microbes Infect. 2009. PMID: 19647801
-
Immunogenicity and efficacy of two types of West Nile virus-like particles different in size and maturation as a second-generation vaccine candidate.Vaccine. 2010 Sep 14;28(40):6588-96. doi: 10.1016/j.vaccine.2010.07.055. Epub 2010 Aug 1. Vaccine. 2010. PMID: 20678586
-
Molecular Determinants of West Nile Virus Virulence and Pathogenesis in Vertebrate and Invertebrate Hosts.Int J Mol Sci. 2020 Nov 30;21(23):9117. doi: 10.3390/ijms21239117. Int J Mol Sci. 2020. PMID: 33266206 Free PMC article. Review.
Cited by
-
In Vitro Infection with Dengue Virus Induces Changes in the Structure and Function of the Mouse Brain Endothelium.PLoS One. 2016 Jun 23;11(6):e0157786. doi: 10.1371/journal.pone.0157786. eCollection 2016. PLoS One. 2016. PMID: 27336851 Free PMC article.
-
Usutu virus and West Nile virus use a transcellular route of neuroinvasion across an in vitro model of the human blood-brain barrier.Npj Viruses. 2024 Jul 25;2(1):32. doi: 10.1038/s44298-024-00034-4. Npj Viruses. 2024. PMID: 40295794 Free PMC article.
-
Antiviral therapy in shrimp through plant virus VLP containing VP28 dsRNA against WSSV.Beilstein J Org Chem. 2021 Jun 1;17:1360-1373. doi: 10.3762/bjoc.17.95. eCollection 2021. Beilstein J Org Chem. 2021. PMID: 34136015 Free PMC article.
-
Development of peptides targeting receptor binding site of the envelope glycoprotein to contain the West Nile virus infection.Sci Rep. 2021 Oct 11;11(1):20131. doi: 10.1038/s41598-021-99696-w. Sci Rep. 2021. PMID: 34635758 Free PMC article.
-
Knowns and unknowns of TiLV-associated neuronal disease.Virulence. 2024 Dec;15(1):2329568. doi: 10.1080/21505594.2024.2329568. Epub 2024 Mar 31. Virulence. 2024. PMID: 38555518 Free PMC article. Review.
References
-
- Cernescu C, Ruta SM, Tardei G, Grancea C, Moldoveanu L, Spulbar E, Tsai T. A high number of severe neurologic clinical forms during an epidemic of West Nile virus infection. Rom J Virol. 1997;48(1-4):13–25. - PubMed
-
- Jamgaonkar AV, Yergolkar PN, Geevarghese G, Joshi GD, Joshi MV, Mishra AC. Serological evidence for Japanese encephalitis virus and West Nile virus infections in water frequenting and terrestrial wild birds in Kolar District, Karnataka State, India. A retrospective study. Acta Virol. 2003;47(3):185–188. - PubMed
-
- Murgue B, Zeller H, Deubel V. The ecology and epidemiology of West Nile virus in Africa, Europe and Asia. Curr Top Microbiol Immunol. 2002;267:195–221. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

