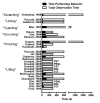
Hypolocomotion, asymmetrically directed behaviors (licking, lifting, flinching, and shaking) and dynamic weight bearing (gait) changes are not measures of neuropathic pain in mice
- PMID: 20529328
- PMCID: PMC2893131
- DOI: 10.1186/1744-8069-6-34
Hypolocomotion, asymmetrically directed behaviors (licking, lifting, flinching, and shaking) and dynamic weight bearing (gait) changes are not measures of neuropathic pain in mice
Abstract
Background: Spontaneous (non-evoked) pain is a major clinical symptom of neuropathic syndromes, one that is understudied in basic pain research for practical reasons and because of a lack of consensus over precisely which behaviors reflect spontaneous pain in laboratory animals. It is commonly asserted that rodents experiencing pain in a hind limb exhibit hypolocomotion and decreased rearing, engage in both reflexive and organized limb directed behaviors, and avoid supporting their body weight on the affected side. Furthermore, it is assumed that the extent of these positive or negative behaviors can be used as a dependent measure of spontaneous chronic pain severity in such animals. In the present study, we tested these assumptions via blinded, systematic observation of digital video of mice with nerve injuries (chronic constriction or spared nerve injury), and automated assessment of locomotor behavior using photocell detection and dynamic weight bearing (i.e., gait) using the CatWalk system.
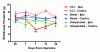
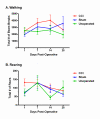
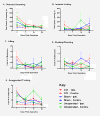
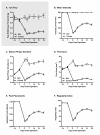
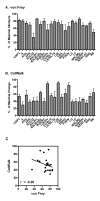
Results: We found no deficits in locomotor activity or rearing associated with neuropathic injury. The frequency of asymmetric (ipsilaterally directed) behaviors were too rare to be seriously considered as representing spontaneous pain, and in any case did not statistically exceed what was blindly observed on the contralateral hind paw and in control (sham operated and unoperated) mice. Changes in dynamic weight bearing, on the other hand, were robust and ipsilateral after spared nerve injury (but not chronic constriction injury). However, we observed timing, pharmacological, and genetic dissociation of mechanical allodynia and gait alterations.
Conclusions: We conclude that spontaneous neuropathic pain in mice cannot be assessed using any of these measures, and thus caution is warranted in making such assertions.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

