Pannexin 2 is expressed by postnatal hippocampal neural progenitors and modulates neuronal commitment
- PMID: 20529862
- PMCID: PMC2915733
- DOI: 10.1074/jbc.M110.130054
Pannexin 2 is expressed by postnatal hippocampal neural progenitors and modulates neuronal commitment
Abstract
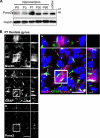
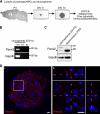
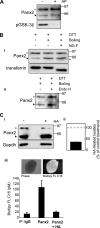
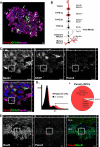
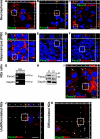
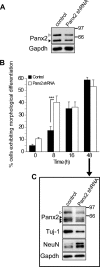
The pannexins (Panx1, -2, and -3) are a mammalian family of putative single membrane channels discovered through homology to invertebrate gap junction-forming proteins, the innexins. Because connexin gap junction proteins are known regulators of neural stem and progenitor cell proliferation, migration, and specification, we asked whether pannexins, specifically Panx2, play a similar role in the postnatal hippocampus. We show that Panx2 protein is differentially expressed by multipotential progenitor cells and mature neurons. Both in vivo and in vitro, Type I and IIa stem-like neural progenitor cells express an S-palmitoylated Panx2 species localizing to Golgi and endoplasmic reticulum membranes. Protein expression is down-regulated during neurogenesis in neuronally committed Type IIb and III progenitor cells and immature neurons. Panx2 is re-expressed by neurons following maturation. Protein expressed by mature neurons is not palmitoylated and localizes to the plasma membrane. To assess the impact of Panx2 on neuronal differentiation, we used short hairpin RNA to suppress Panx2 expression in Neuro2a cells. Knockdown significantly accelerated the rate of neuronal differentiation. Neuritic extension and the expression of antigenic markers of mature neurons occurred earlier in stable lines expressing Panx2 short hairpin RNA than in controls. Together, these findings describe an endogenous post-translational regulation of Panx2, specific to early neural progenitor cells, and demonstrate that this expression plays a role in modulating the timing of their commitment to a neuronal lineage.
Figures

References
-
- Kempermann G., Jessberger S., Steiner B., Kronenberg G. (2004) Trends Neurosci. 27, 447–452 - PubMed
-
- Thompson A., Boekhoorn K., Van Dam A. M., Lucassen P. J. (2008) Genes Brain Behav. 7, Suppl. 1, 28–42 - PubMed
-
- D'Ascenzo M., Piacentini R., Casalbore P., Budoni M., Pallini R., Azzena G. B., Grassi C. (2006) Eur. J. Neurosci. 23, 935–944 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

