Ubiquilin functions in autophagy and is degraded by chaperone-mediated autophagy
- PMID: 20529957
- PMCID: PMC2908472
- DOI: 10.1093/hmg/ddq231
Ubiquilin functions in autophagy and is degraded by chaperone-mediated autophagy
Abstract
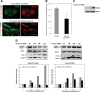
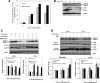
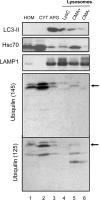
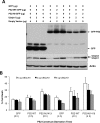
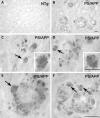
Autophagy is the process by which organelles and portions of the cytoplasm are degraded in lysosomes. Several different forms of autophagy are known that are distinguishable chiefly by the mode in which cargo is delivered to the lysosome for degradation. Ubiquilin was recently reported to regulate macroautophagy, the form of autophagy in which cytosolic cargo is packaged in a double-membrane structure or autophagosome that fuses with lysosomes for degradation. We confirm here using different morphological and biochemical procedures that ubiquilin is present in autophagosomes in HeLa cells and in brain and liver tissue of mouse. Coimmunoprecipitation studies indicated that ubiquilin binds the autophagosome marker LC3 in a complex and that reduction of ubiquilin expression reduces autophagosome formation, which correlates with a reduction in maturation of LC3-I to the LC3-II form of the protein. We found that ubiquilin is degraded during both macroautophagy and during chaperone-mediated autophagy (CMA), the latter of which involves the active transport of proteins into lysosomes. We discuss the implication of this degradation in mediating cross-talk between macroautophagy and CMA. Finally, we demonstrate that ubiquilin protects cells against starvation-induced cell death propagated by overexpression of mutant Alzheimer's disease PS2N141I protein and green fluorescent protein (GFP)-huntingtin exon-1 fusion protein containing 74 polyglutamines.
Figures





References
-
- Wu A.L., Wang J., Zheleznyak A., Brown E.J. Ubiquitin-related proteins regulate interaction of vimentin intermediate filaments with the plasma membrane. Mol. Cell. 1999;4:619–625. doi:10.1016/S1097-2765(00)80212-9. - DOI - PubMed
-
- Mah A.L., Perry G., Smith M.A., Monteiro M.J. Identification of ubiquilin, a novel presenilin interactor that increases presenilin protein accumulation. J. Cell Biol. 2000;151:847–862. doi:10.1083/jcb.151.4.847. - DOI - PMC - PubMed
-
- Kleijnen M.F., Shih A.H., Zhou P., Kumar S., Soccio R.E., Kedersha N.L., Gill G., Howley P.M. The hPLIC proteins may provide a link between the ubiquitination machinery and the proteasome. Mol. Cell. 2000;6:409–419. doi:10.1016/S1097-2765(00)00040-X. - DOI - PubMed
-
- Miller J., Gordon C. The regulation of proteasome degradation by multi-ubiquitin chain binding proteins. FEBS Lett. 2005;579:3224–3230. doi:10.1016/j.febslet.2005.03.042. - DOI - PubMed
-
- Elsasser S., Finley D. Delivery of ubiquitinated substrates to protein-unfolding machines. Nat. Cell Biol. 2005;7:742–749. doi:10.1038/ncb0805-742. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

