Targeted deletions of cyclooxygenase-2 and atherogenesis in mice
- PMID: 20530000
- PMCID: PMC2909762
- DOI: 10.1161/CIRCULATIONAHA.109.910687
Targeted deletions of cyclooxygenase-2 and atherogenesis in mice
Abstract
Background: Although the dominant product of vascular Cyclooxygenase-2 (COX-2), prostacyclin (PGI(2)), restrains atherogenesis, inhibition and deletion of COX-2 have yielded conflicting results in mouse models of atherosclerosis. Floxed mice were used to parse distinct cellular contributions of COX-2 in macrophages and T cells (TCs) to atherogenesis.
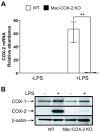
Methods and results: Deletion of macrophage-COX-2 (Mac-COX-2KOs) was attained with LysMCre mice and completely suppressed lipopolysaccharide-stimulated macrophage prostaglandin (PG) formation and lipopolysaccharide-evoked systemic PG biosynthesis by approximately 30%. Lipopolysaccharide-stimulated COX-2 expression was suppressed in polymorphonuclear leukocytes isolated from MacKOs, but PG formation was not even detected in polymorphonuclear leukocyte supernatants from control mice. Atherogenesis was attenuated when MacKOs were crossed into hyperlipidemic low-density lipoprotein receptor knockouts. Deletion of Mac-COX-2 appeared to remove a restraint on COX-2 expression in lesional nonleukocyte (CD45- and CD11b-negative) vascular cells that express vascular cell adhesion molecule and variably alpha-smooth muscle actin and vimentin, portending a shift in PG profile and consequent atheroprotection. Basal expression of COX-2 was minimal in TCs, but use of CD4Cre to generate TC knockouts depressed its modest upregulation by anti-CD3epsilon. However, biosynthesis of PGs, TC composition in lymphatic organs, and atherogenesis in low-density lipoprotein receptor knockouts were unaltered in TC knockouts.
Conclusions: Macrophage-COX-2, primarily a source of thromboxane A(2) and prostaglandin (PG)E(2), promotes atherogenesis and exerts a restraint on enzyme expression by lesional cells suggestive of vascular smooth muscle cells, a prominent source of atheroprotective prostacyclin. TC COX-2 does not detectably influence TC development or function or atherogenesis in mice.
Conflict of interest statement
Figures
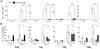
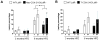


Similar articles
-
Cyclooxygenase-2 in endothelial and vascular smooth muscle cells restrains atherogenesis in hyperlipidemic mice.Circulation. 2014 Apr 29;129(17):1761-9. doi: 10.1161/CIRCULATIONAHA.113.007913. Epub 2014 Feb 11. Circulation. 2014. PMID: 24519928 Free PMC article.
-
Acceleration of atherogenesis by COX-1-dependent prostanoid formation in low density lipoprotein receptor knockout mice.Proc Natl Acad Sci U S A. 2001 Mar 13;98(6):3358-63. doi: 10.1073/pnas.061607398. Epub 2001 Mar 6. Proc Natl Acad Sci U S A. 2001. PMID: 11248083 Free PMC article.
-
Disruption of the 5-lipoxygenase pathway attenuates atherogenesis consequent to COX-2 deletion in mice.Proc Natl Acad Sci U S A. 2012 Apr 24;109(17):6727-32. doi: 10.1073/pnas.1115313109. Epub 2012 Apr 9. Proc Natl Acad Sci U S A. 2012. PMID: 22493243 Free PMC article.
-
Deletion of microsomal prostaglandin E synthase-1 augments prostacyclin and retards atherogenesis.Proc Natl Acad Sci U S A. 2006 Sep 26;103(39):14507-12. doi: 10.1073/pnas.0606586103. Epub 2006 Sep 14. Proc Natl Acad Sci U S A. 2006. PMID: 16973753 Free PMC article.
-
Cyclooxygenase-2 and inflammation in atherosclerosis.Curr Opin Pharmacol. 2004 Apr;4(2):116-23. doi: 10.1016/j.coph.2003.12.003. Curr Opin Pharmacol. 2004. PMID: 15063354 Review.
Cited by
-
Subacute treatment of carprofen facilitate splenocardiac resolution deficit in cardiac injury.J Leukoc Biol. 2018 Dec;104(6):1173-1186. doi: 10.1002/JLB.3A0618-223R. Epub 2018 Aug 26. J Leukoc Biol. 2018. PMID: 30145840 Free PMC article.
-
Cyclooxygenase-2 in endothelial and vascular smooth muscle cells restrains atherogenesis in hyperlipidemic mice.Circulation. 2014 Apr 29;129(17):1761-9. doi: 10.1161/CIRCULATIONAHA.113.007913. Epub 2014 Feb 11. Circulation. 2014. PMID: 24519928 Free PMC article.
-
Current Medical Therapy and Revascularization in Peripheral Artery Disease of the Lower Limbs: Impacts on Subclinical Chronic Inflammation.Int J Mol Sci. 2023 Nov 8;24(22):16099. doi: 10.3390/ijms242216099. Int J Mol Sci. 2023. PMID: 38003290 Free PMC article. Review.
-
Anti-inflammatory therapies for atherosclerosis.Nat Rev Cardiol. 2015 Apr;12(4):199-211. doi: 10.1038/nrcardio.2015.5. Epub 2015 Feb 10. Nat Rev Cardiol. 2015. PMID: 25666404 Review.
-
Celecoxib-Loaded Electrospun Fibrous Antiadhesion Membranes Reduce COX-2/PGE2 Induced Inflammation and Epidural Fibrosis in a Rat Failed Back Surgery Syndrome Model.Neural Plast. 2021 Feb 23;2021:6684176. doi: 10.1155/2021/6684176. eCollection 2021. Neural Plast. 2021. PMID: 33679970 Free PMC article.
References
-
- Smyth E, Burke A, FitzGerald GA. Lipid-derived autocoids. In: Hardman JG, Limbird LE, Gilman AG, editors. Goodman & Gilman's The Pharmacological Basis of Therapeutics. McGraw-Hill; New York: 2005. pp. 653–670.
-
- Grosser T, Yu Y, FitzGerald GA. Emotion recollected in tranquility; lessons from the COX-2 saga. Ann Rev Med. 2010;61:17–33. - PubMed
-
- Kobayashi T, Tahara Y, Matsumoto M, Iguchi M, Sano H, Murayama T, Arai H, Oida H, Yurugi-Kobayashi T, Yamashita JK, Katagiri H, Majima M, Yokode M, Kita T, Narumiya S. Roles of thromboxane A(2) and prostacyclin in the development of atherosclerosis in apoE-deficient mice. J Clin Invest. 2004;114:784–794. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

