Length dependence of force generation exhibit similarities between rat cardiac myocytes and skeletal muscle fibres
- PMID: 20530113
- PMCID: PMC2956905
- DOI: 10.1113/jphysiol.2010.190504
Length dependence of force generation exhibit similarities between rat cardiac myocytes and skeletal muscle fibres
Abstract
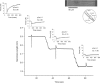
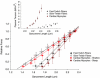
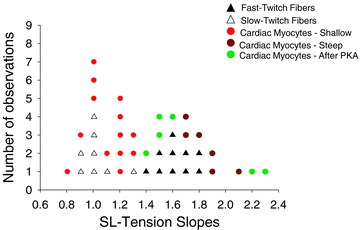
According to the Frank-Starling relationship, increased ventricular volume increases cardiac output, which helps match cardiac output to peripheral circulatory demand. The cellular basis for this relationship is in large part the myofilament length-tension relationship. Length-tension relationships in maximally calcium activated preparations are relatively shallow and similar between cardiac myocytes and skeletal muscle fibres. During twitch activations length-tension relationships become steeper in both cardiac and skeletal muscle; however, it remains unclear whether length dependence of tension differs between striated muscle cell types during submaximal activations. The purpose of this study was to compare sarcomere length-tension relationships and the sarcomere length dependence of force development between rat skinned left ventricular cardiac myocytes and fast-twitch and slow-twitch skeletal muscle fibres. Muscle cell preparations were calcium activated to yield 50% maximal force, after which isometric force and rate constants (k(tr)) of force development were measured over a range of sarcomere lengths. Myofilament length-tension relationships were considerably steeper in fast-twitch fibres compared to slow-twitch fibres. Interestingly, cardiac myocyte preparations exhibited two populations of length-tension relationships, one steeper than fast-twitch fibres and the other similar to slow-twitch fibres. Moreover, myocytes with shallow length-tension relationships were converted to steeper length-tension relationships by protein kinase A (PKA)-induced myofilament phosphorylation. Sarcomere length-k(tr) relationships were distinct between all three cell types and exhibited patterns markedly different from Ca(2+) activation-dependent k(tr) relationships. Overall, these findings indicate cardiac myocytes exhibit varied length-tension relationships and sarcomere length appears a dominant modulator of force development rates. Importantly, cardiac myocyte length-tension relationships appear able to switch between slow-twitch-like and fast-twitch-like by PKA-mediated myofibrillar phosphorylation, which implicates a novel means for controlling Frank-Starling relationships.
Figures



References
-
- Bardswell SC, Kentish J. Resting and active sarcomere lengths in isolated rat ventricular myocytes. Biophys J. 2003;84:431a.
-
- Braunwald E, Ross J. Applicability of Starling's law of the heart to man. Circ Res. 1964;Suppl ii:169–181. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

