Allosteric inhibition of the regulator of G protein signaling-Galpha protein-protein interaction by CCG-4986
- PMID: 20530129
- PMCID: PMC2939487
- DOI: 10.1124/mol.109.063388
Allosteric inhibition of the regulator of G protein signaling-Galpha protein-protein interaction by CCG-4986
Abstract
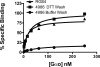
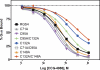
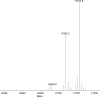
Regulator of G protein signaling (RGS) proteins act to temporally modulate the activity of G protein subunits after G protein-coupled receptor activation. RGS proteins exert their effect by directly binding to the activated Galpha subunit of the G protein, catalyzing the accelerated hydrolysis of GTP and returning the G protein to its inactive, heterotrimeric form. In previous studies, we have sought to inhibit this GTPase-accelerating protein activity of the RGS protein by using small molecules. In this study, we investigated the mechanism of CCG-4986 [methyl-N-[(4-chlorophenyl)sulfonyl]-4-nitro-benzenesulfinimidoate], a previously reported small-molecule RGS inhibitor. Here, we find that CCG-4986 inhibits RGS4 function through the covalent modification of two spatially distinct cysteine residues on RGS4. We confirm that modification of Cys132, located near the RGS/Galpha interaction surface, modestly inhibits Galpha binding and GTPase acceleration. In addition, we report that modification of Cys148, a residue located on the opposite face of RGS4, can disrupt RGS/Galpha interaction through an allosteric mechanism that almost completely inhibits the Galpha-RGS protein-protein interaction. These findings demonstrate three important points: 1) the modification of the Cys148 allosteric site results in significant changes to the RGS interaction surface with Galpha; 2) this identifies a "hot spot" on RGS4 for binding of small molecules and triggering an allosteric change that may be significantly more effective than targeting the actual protein-protein interaction surface; and 3) because of the modification of a positional equivalent of Cys148 in RGS8 by CCG-4986, lack of inhibition indicates that RGS proteins exhibit fundamental differences in their responses to small-molecule ligands.
Figures
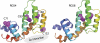


Similar articles
-
The RGS protein inhibitor CCG-4986 is a covalent modifier of the RGS4 Galpha-interaction face.Biochim Biophys Acta. 2007 Sep;1774(9):1213-20. doi: 10.1016/j.bbapap.2007.06.002. Epub 2007 Jun 29. Biochim Biophys Acta. 2007. PMID: 17660054 Free PMC article.
-
Reversible, allosteric small-molecule inhibitors of regulator of G protein signaling proteins.Mol Pharmacol. 2010 Sep;78(3):524-33. doi: 10.1124/mol.110.065128. Epub 2010 Jun 22. Mol Pharmacol. 2010. PMID: 20571077 Free PMC article.
-
A nanomolar-potency small molecule inhibitor of regulator of G-protein signaling proteins.Biochemistry. 2011 Apr 19;50(15):3181-92. doi: 10.1021/bi1019622. Epub 2011 Mar 29. Biochemistry. 2011. PMID: 21329361 Free PMC article.
-
Role of palmitoylation in RGS protein function.Methods Enzymol. 2004;389:33-55. doi: 10.1016/S0076-6879(04)89003-7. Methods Enzymol. 2004. PMID: 15313558 Review.
-
Fluorescence-based assays for RGS box function.Methods Enzymol. 2004;389:56-71. doi: 10.1016/S0076-6879(04)89004-9. Methods Enzymol. 2004. PMID: 15313559 Review.
Cited by
-
Gα(i2)-mediated protection from ischaemic injury is modulated by endogenous RGS proteins in the mouse heart.Cardiovasc Res. 2011 Jul 1;91(1):45-52. doi: 10.1093/cvr/cvr054. Epub 2011 Feb 23. Cardiovasc Res. 2011. PMID: 21349876 Free PMC article.
-
Modification and functional inhibition of regulator of G-protein signaling 4 (RGS4) by 4-hydroxy-2-nonenal.Chem Res Toxicol. 2013 Dec 16;26(12):1832-9. doi: 10.1021/tx400212q. Epub 2013 Nov 14. Chem Res Toxicol. 2013. PMID: 24229325 Free PMC article.
-
Reversible inhibitors of regulators of G-protein signaling identified in a high-throughput cell-based calcium signaling assay.Cell Signal. 2013 Dec;25(12):2848-55. doi: 10.1016/j.cellsig.2013.09.007. Epub 2013 Sep 14. Cell Signal. 2013. PMID: 24041654 Free PMC article.
-
Differential Protein Dynamics of Regulators of G-Protein Signaling: Role in Specificity of Small-Molecule Inhibitors.J Am Chem Soc. 2018 Mar 7;140(9):3454-3460. doi: 10.1021/jacs.7b13778. Epub 2018 Feb 26. J Am Chem Soc. 2018. PMID: 29460621 Free PMC article.
-
Regulators of G-protein signaling and their Gα substrates: promises and challenges in their use as drug discovery targets.Pharmacol Rev. 2011 Sep;63(3):728-49. doi: 10.1124/pr.110.003038. Epub 2011 Jul 7. Pharmacol Rev. 2011. PMID: 21737532 Free PMC article. Review.
References
-
- Arkin MR, Wells JA. (2004) Small-molecule inhibitors of protein-protein interactions: progressing towards the dream. Nat Rev Drug Discov 3:301–317 - PubMed
-
- Berman DM, Kozasa T, Gilman AG. (1996a) The GTPase-activating protein RGS4 stabilizes the transition state for nucleotide hydrolysis. J Biol Chem 271:27209–27212 - PubMed
-
- Berman DM, Wilkie TM, Gilman AG. (1996b) GAIP and RGS4 are GTPase-activating proteins for the Gi subfamily of G protein α subunits. Cell 86:445–452 - PubMed
-
- Blazer LL, Neubig RR. (2009) Small molecule protein-protein interaction inhibitors as CNS therapeutic agents: current progress and future hurdles. Neuropsychopharmacology 34:126–141 - PubMed
-
- Gilman AG. (1987) G proteins: transducers of receptor-generated signals. Annu Rev Biochem 56:615–649 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

