Epidermis-type lipoxygenase 3 regulates adipocyte differentiation and peroxisome proliferator-activated receptor gamma activity
- PMID: 20530198
- PMCID: PMC2916447
- DOI: 10.1128/MCB.01806-08
Epidermis-type lipoxygenase 3 regulates adipocyte differentiation and peroxisome proliferator-activated receptor gamma activity
Abstract
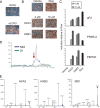
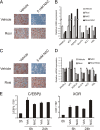
The nuclear receptor peroxisome proliferator-activated receptor gamma (PPAR gamma) is essential for adipogenesis. Although several fatty acids and their derivatives are known to bind and activate PPAR gamma, the nature of the endogenous ligand(s) promoting the early stages of adipocyte differentiation has remained enigmatic. Previously, we showed that lipoxygenase (LOX) activity is involved in activation of PPAR gamma during the early stages of adipocyte differentiation. Of the seven known murine LOXs, only the unconventional LOX epidermis-type lipoxygenase 3 (eLOX3) is expressed in 3T3-L1 preadipocytes. Here, we show that forced expression of eLOX3 or addition of eLOX3 products stimulated adipogenesis under conditions that normally require an exogenous PPAR gamma ligand for differentiation. Hepoxilins, a group of oxidized arachidonic acid derivatives produced by eLOX3, bound to and activated PPAR gamma. Production of hepoxilins was increased transiently during the initial stages of adipogenesis. Furthermore, small interfering RNA-mediated or retroviral short hairpin RNA-mediated knockdown of eLOX3 expression abolished differentiation of 3T3-L1 preadipocytes. Finally, we demonstrate that xanthine oxidoreductase (XOR) and eLOX3 synergistically enhanced PPAR gamma-mediated transactivation. Collectively, our results indicate that hepoxilins produced by the concerted action of XOR and eLOX3 may function as PPAR gamma activators capable of promoting the early PPAR gamma-dependent steps in the conversion of preadipocytes into adipocytes.
Figures






References
-
- Araujo, P., Z.-Y. Du, T.-T. Nguyen, and E. Holen. 2008. Direct injection of redissolved cell culture media into a single-column liquid chromatography coupled to mass spectrometry for the measurement of PGE2. Open Anal. Chem. J. 2:62-66.
-
- Barak, Y., M. C. Nelson, E. S. Ong, Y. Z. Jones, P. Ruiz-Lozano, K. R. Chien, A. Koder, and R. M. Evans. 1999. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol. Cell 4:585-595. - PubMed
-
- Bastie, C., D. Holst, D. Gaillard, C. Jehl-Pietri, and P. A. Grimaldi. 1999. Expression of peroxisome proliferator-activated receptor PPARdelta promotes induction of PPARγ and adipocyte differentiation in 3T3C2 fibroblasts. J. Biol. Chem. 274:21920-21925. - PubMed
-
- Bastie, C., S. Luquet, D. Holst, C. Jehl-Pietri, and P. A. Grimaldi. 2000. Alterations of peroxisome proliferator-activated receptor delta activity affect fatty acid-controlled adipose differentiation. J. Biol. Chem. 275:38768-38773. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
