Structural insights into the recognition mechanism between an antitumor galectin AAL and the Thomsen-Friedenreich antigen
- PMID: 20530247
- PMCID: PMC3229422
- DOI: 10.1096/fj.10-159111
Structural insights into the recognition mechanism between an antitumor galectin AAL and the Thomsen-Friedenreich antigen
Abstract
Thomsen-Friedenreich (TF) antigen, which plays an important role in the regulation of cancer cell proliferation, occurs in ∼90% of all human cancers and precancerous conditions. Although TF antigen has been known for almost 80 yr as a pancarcinoma antigen, the recognition mechanism between TF antigen and target protein has not been structurally characterized. A number of studies indicated that TF disaccharide is a potential ligand of the galactoside-binding galectins. In this work, we identified the TF antigen as a potential ligand of the antitumor galectin AAL (Agrocybe aegerita lectin) through glycan array analysis and reported the crystal structure of AAL complexed with the TF antigen. The structure provides a first look at the recognition mode between AAL and TF antigen, which is unique in a conservative (Glu-water-Arg-water) structural motif-based hydrogen bond network. Structure-based mutagenesis analysis further revealed the residues responsible for recognition specificity and binding affinity. Crystal structures of AAL complexed with two other TF-containing glycans showed that the unique TF recognition mode is kept intact, which may be commonly adopted in some cancer-related galectins. The finding provided the new target and approach for the antitumor drug design and relative strategy based on the AAL-TF recognition mode as a prototype model.
Figures


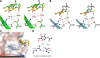

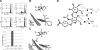
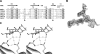
References
-
- Baldus S. E., Engelmann K., Hanisch F.-G. (2004) MUC1 and the MUCs: a family of human mucins with impact in cancer biology. Crit. Rev. Clin. Lab. Sci. 41, 189–231 - PubMed
-
- Hanisch F. G. B. S. (1997) The Thomsen-Friedenreich (TF) antigen: a critical review on the structural, biosynthetic and histochemical aspects of a pancarcinoma-associated antigen. Histol. Histopathol. 12, 263–281 - PubMed
-
- Yu L.-G. (2007) The oncofetal Thomsen–Friedenreich carbohydrate antigen in cancer progression. Glycoconj. J. 24, 411–420 - PubMed
-
- Springer G. F. (1997) Immunoreactive T and Tn epitopes in cancer diagnosis, prognosis, and immunotherapy. J. Mol. Med. 75, 594–602 - PubMed
-
- Slovin S.F., Musselli G. R., Fernandez C., Diani M., Verbel D., Danishefsky S., Livingston P., Scher H. I. (2005) Thomsen-Friedenreich (TF) antigen as a target for prostate cancer vaccine: clinical trial results with TF cluster (c) -KLH plus QS21 conjugate vaccine in patients with biochemically relapsed prostate cancer Cancer Immunol. Immunother. 54, 694–702 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

