An evaluation of the role of properdin in alternative pathway activation on Neisseria meningitidis and Neisseria gonorrhoeae
- PMID: 20530262
- PMCID: PMC2933790
- DOI: 10.4049/jimmunol.0903598
An evaluation of the role of properdin in alternative pathway activation on Neisseria meningitidis and Neisseria gonorrhoeae
Abstract
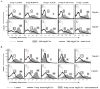
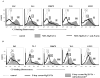
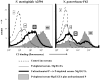
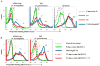
Properdin, a positive regulator of the alternative pathway (AP) of complement is important in innate immune defenses against invasive neisserial infections. Recently, commercially available unfractionated properdin was shown to bind to certain biological surfaces, including Neisseria gonorrhoeae, which facilitated C3 deposition. Unfractionated properdin contains aggregates or high-order oligomers, in addition to its physiological "native" (dimeric, trimeric, and tetrameric) forms. We examined the role of properdin in AP activation on diverse strains of Neisseria meningitidis and N. gonorrhoeae specifically using native versus unfractionated properdin. C3 deposition on Neisseria decreased markedly when properdin function was blocked using an anti-properdin mAb or when properdin was depleted from serum. Maximal AP-mediated C3 deposition on Neisseriae even at high (80%) serum concentrations required properdin. Consistent with prior observations, preincubation of bacteria with unfractionated properdin, followed by the addition of properdin-depleted serum resulted in higher C3 deposition than when bacteria were incubated with properdin-depleted serum alone. Unexpectedly, none of 10 Neisserial strains tested bound native properdin. Consistent with its inability to bind to Neisseriae, preincubating bacteria with native properdin followed by the addition of properdin-depleted serum did not cause detectable increases in C3 deposition. However, reconstituting properdin-depleted serum with native properdin a priori enhanced C3 deposition on all strains of Neisseria tested. In conclusion, the physiological forms of properdin do not bind directly to either N. meningitidis or N. gonorrhoeae but play a crucial role in augmenting AP-dependent C3 deposition on the bacteria through the "conventional" mechanism of stabilizing AP C3 convertases.
Figures


References
-
- Pangburn MK. Analysis of the natural polymeric forms of human properdin and their functions in complement activation. J Immunol. 1989;142:202–207. - PubMed
-
- Smith CA, Pangburn MK, Vogel CW, Muller-Eberhard HJ. Molecular architecture of human properdin, a positive regulator of the alternative pathway of complement. JBiol Chem. 1984;259:4582–4588. - PubMed
-
- Pillemer L, Blum L, Lepow IH, Ross OA, Todd EW, Wardlaw AC. The properdin system and immunity. I. Demonstration and isolation of a new serum protein, properdin, and its role in immune phenomena. Science. 1954;120:279–285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

