CD8+ T cells specific for immunodominant trans-sialidase epitopes contribute to control of Trypanosoma cruzi infection but are not required for resistance
- PMID: 20530265
- PMCID: PMC3784248
- DOI: 10.4049/jimmunol.1000432
CD8+ T cells specific for immunodominant trans-sialidase epitopes contribute to control of Trypanosoma cruzi infection but are not required for resistance
Abstract
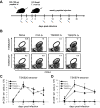
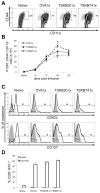
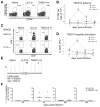
CD8(+) T cells are essential for controlling Trypanosoma cruzi infection. During Brazil strain infection, C57BL/6 mice expand parasite-specific CD8(+) T cells recognizing the dominant TSKB20 (ANYKFTLV) and subdominant TSKB74 (VNYDFTLV) trans-sialidase gene (TS)-encoded epitopes with up to 40% of all CD8(+) T cells specific for these epitopes. Although this is one of the largest immunodominant T cell responses described for any infection, most mice fail to clear T. cruzi and subsequently develop chronic disease. To determine if immunodominant TS-specific CD8(+) T cells are necessary for resistance to infection, we epitope-tolerized mice by high-dose i.v. injections of TSKB20 or TSKB74 peptides. Tolerance induction led to deletion of TS-specific CD8(+) T cells but did not prevent the expansion of other effector CD8(+) T cell populations. Mice tolerized against either TSKB20 or TSKB74, or both epitopes simultaneously, exhibited transient increases in parasite loads, although ultimately they controlled the acute infection. Furthermore, BALB/c mice tolerized against the TSKD14 peptide effectively controlled acute T. cruzi infection. These data are consistent with the hypothesis that development of high-frequency CD8(+) T cell populations focused on TS-derived epitopes contributes to optimal control of acute infection but is not required for the development of immune resistance.
Figures


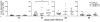

References
-
- Wong P, Pamer EG. CD8 T cell responses to infectious pathogens. Annu Rev Immunol. 2003;21:29–70. - PubMed
-
- Harty JT, Tvinnereim AR, White DW. CD8+ T cell effector mechanisms in resistance to infection. Annu Rev Immunol. 2000;18:275–308. - PubMed
-
- Yewdell JW, Bennink JR. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu Rev Immunol. 1999;17:51–88. - PubMed
-
- Pamer EG. Immune responses to Listeria monocytogenes. Nat Rev Immunol. 2004;4:812–823. - PubMed
-
- Yewdell JW. Confronting complexity: real-world immunodominance in antiviral CD8+ T cell responses. Immunity. 2006;25:533–543. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

