One enzyme, two functions: PON2 prevents mitochondrial superoxide formation and apoptosis independent from its lactonase activity
- PMID: 20530481
- PMCID: PMC2915675
- DOI: 10.1074/jbc.M110.118604
One enzyme, two functions: PON2 prevents mitochondrial superoxide formation and apoptosis independent from its lactonase activity
Abstract
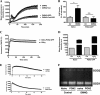
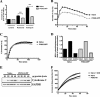
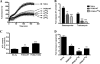
The human enzyme paraoxonase-2 (PON2) has two functions, an enzymatic lactonase activity and the reduction of intracellular oxidative stress. As a lactonase, it dominantly hydrolyzes bacterial signaling molecule 3OC12 and may contribute to the defense against pathogenic Pseudomonas aeruginosa. By its anti-oxidative effect, PON2 reduces cellular oxidative damage and influences redox signaling, which promotes cell survival. This may be appreciated but also deleterious given that high PON2 levels reduce atherosclerosis but may stabilize tumor cells. Here we addressed the unknown mechanisms and linkage of PON2 enzymatic and anti-oxidative function. We demonstrate that PON2 indirectly but specifically reduced superoxide release from the inner mitochondrial membrane, irrespective whether resulting from complex I or complex III of the electron transport chain. PON2 left O(2)(-) dismutase activities and cytochrome c expression unaltered, and it did not oxidize O(2)(-) but rather prevented its formation, which implies that PON2 acts by modulating quinones. To analyze linkage to hydrolytic activity, we introduced several point mutations and show that residues His(114) and His(133) are essential for PON2 activity. Further, we mapped its glycosylation sites and provide evidence that glycosylation, but not a native polymorphism Ser/Cys(311), was critical to its activity. Importantly, none of these mutations altered the anti-oxidative/anti-apoptotic function of PON2, demonstrating unrelated activities of the same protein. Collectively, our study provides detailed mechanistic insight into the functions of PON2, which is important for its role in innate immunity, atherosclerosis, and cancer.
Figures
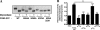
References
-
- Madamanchi N. R., Vendrov A., Runge M. S. (2005) Arterioscler. Thromb. Vasc. Biol. 25, 29–38 - PubMed
-
- Landriscina M., Maddalena F., Laudiero G., Esposito F. (2009) Antioxid. Redox Signal. 11, 2701–2716 - PubMed
-
- Gogvadze V., Orrenius S., Zhivotovsky B. (2009) Apoptosis 14, 624–640 - PubMed
-
- Orrenius S., Zhivotovsky B. (2005) Nat. Chem. Biol. 1, 188–189 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

