Evaluation of calcium acetate/magnesium carbonate as a phosphate binder compared with sevelamer hydrochloride in haemodialysis patients: a controlled randomized study (CALMAG study) assessing efficacy and tolerability
- PMID: 20530499
- PMCID: PMC2957591
- DOI: 10.1093/ndt/gfq292
Evaluation of calcium acetate/magnesium carbonate as a phosphate binder compared with sevelamer hydrochloride in haemodialysis patients: a controlled randomized study (CALMAG study) assessing efficacy and tolerability
Abstract
Background: Phosphate binders are required to control serum phosphorus in dialysis patients. A phosphate binder combining calcium and magnesium offers an interesting therapeutic option.
Methods: This controlled randomized, investigator-masked, multicentre trial investigated the effect of calcium acetate/magnesium carbonate (CaMg) on serum phosphorus levels compared with sevelamer hydrochloride (HCl). The study aim was to show non-inferiority of CaMg in lowering serum phosphorus levels into Kidney Disease Outcome Quality Initiative (K/DOQI) target level range after 24 weeks. Three hundred and twenty-six patients from five European countries were included. After a phosphate binder washout period, 255 patients were randomized in a 1:1 fashion. Two hundred and four patients completed the study per protocol (CaMg, N = 105; dropouts N = 18; sevelamer-HCl, N = 99; dropouts N = 34). Patient baseline characteristics were similar in both groups.
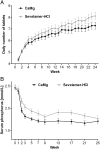
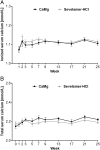
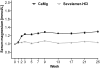
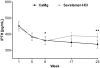
Results: Serum phosphorus levels had decreased significantly with both drugs at week 25, and the study hypothesis of CaMg not being inferior to sevelamer-HCl was confirmed. The area under the curve for serum phosphorus (P = 0.0042) and the number of visits above K/DOQI (≤1.78 mmol/L, P = 0.0198) and Kidney disease: Improving global outcomes (KDIGO) targets (≤1.45 mmol/L, P = 0.0067) were significantly lower with CaMg. Ionized serum calcium did not differ between groups; total serum calcium increased in the CaMg group (treatment difference 0.0477 mmol/L; P = 0.0032) but was not associated with a higher risk of hypercalcaemia. An asymptomatic increase in serum magnesium occurred in CaMg-treated patients (treatment difference 0.2597 mmol/L, P < 0.0001). There was no difference in the number of patients with adverse events.
Conclusion: CaMg was non-inferior to the comparator at controlling serum phosphorus levels at Week 25. There was no change in ionized calcium; there was minimal increase in total serum calcium and a small increase in serum magnesium. It had a good tolerability profile and thus may represent an effective treatment of hyperphosphataemia.
Figures
Comment in
-
Is combined calcium/magnesium phosphate binder really noninferior to sevelamer hydrochloride?Nephrol Dial Transplant. 2011 Apr;26(4):1442-3; author reply 1443-4. doi: 10.1093/ndt/gfq778. Epub 2011 Feb 21. Nephrol Dial Transplant. 2011. PMID: 21339309 No abstract available.
References
-
- Block GA, Hulbert-Shearon TE, Levin NW, et al. Association of serum phosphorus and calcium × phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31:607–617. - PubMed
-
- Covic A, Kothawala P, Bernal M, et al. Systematic review of the evidence underlying the association between mineral metabolism disturbances and risk of all-cause mortality, cardiovascular mortality and cardiovascular events in chronic kidney disease. Nephrol Dial Transplant. 2009;24:1506–1523. - PubMed
-
- Lezaic V, Tirmenstajn-Jankovic B, Bukvic D, et al. Efficacy of hyperphosphatemia control in the progression of chronic renal failure and the prevalence of cardiovascular calcification. Clin Nephrol. 2009;71:21–29. - PubMed
-
- Wald R, Sarnak MJ, Tighiouart H, et al. Disordered mineral metabolism in hemodialysis patients: an analysis of cumulative effects in the Hemodialysis (HEMO) Study. Am J Kidney Dis. 2008;52:531–540. - PubMed
-
- Tentori F, Blayney MJ, Albert JM, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2008;52:519–530. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

