Netrin-guided accessory cell morphogenesis dictates the dendrite orientation and migration of a Drosophila sensory neuron
- PMID: 20530550
- PMCID: PMC2882139
- DOI: 10.1242/dev.047795
Netrin-guided accessory cell morphogenesis dictates the dendrite orientation and migration of a Drosophila sensory neuron
Abstract
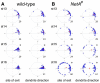
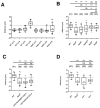
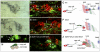
Accessory cells, which include glia and other cell types that develop in close association with neurons, have been shown to play key roles in regulating neuron development. However, the underlying molecular and cellular mechanisms remain poorly understood. A particularly intimate association between accessory cells and neurons is found in insect chordotonal organs. We have found that the cap cell, one of two accessory cells of v'ch1, a chordotonal organ in the Drosophila embryo, strongly influences the development of its associated neuron. As it projects a long dorsally directed cellular extension, the cap cell reorients the dendrite of the v'ch1 neuron and tows its cell body dorsally. Cap cell morphogenesis is regulated by Netrin-A, which is produced by epidermal cells at the destination of the cap cell process. In Netrin-A mutant embryos, the cap cell forms an aberrant, ventrally directed process. As the cap cell maintains a close physical connection with the tip of the dendrite, the latter is dragged into an abnormal position and orientation, and the neuron fails to undergo its normal dorsal migration. Misexpression of Netrin-A in oenocytes, secretory cells that lie ventral to the cap cell, leads to aberrant cap cell morphogenesis, suggesting that Netrin-A acts as an instructive cue to direct the growth of the cap cell process. The netrin receptor Frazzled is required for normal cap cell morphogenesis, and mutant rescue experiments indicate that it acts in a cell-autonomous fashion.
Figures




References
-
- Bradford D., Cole S. J., Cooper H. M. (2009). Netrin-1: diversity in development. Int. J. Biochem. Cell Biol. 41, 487-493 - PubMed
-
- Campos-Ortega J. A., Hartenstein V. (1985). The embryonic development of Drosophila melanogaster Berlin: Springer Verlag;
-
- Carlson S. D., Hilgers S. L., Juang J. L. (1997a). First developmental signs of the scolopale (glial) cell and neuron comprising the chordotonal organ in the Drosophila embryo. Glia 19, 269-274 - PubMed
-
- Carlson S. D., Hilgers S. L., Juang J. L. (1997b). Ultrastructure and blood-nerve barrier of chordotonal organs in the Drosophila embryo. J. Neurocytol. 26, 377-388 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

