Efficacy of standardised manual therapy and home exercise programme for chronic rotator cuff disease: randomised placebo controlled trial
- PMID: 20530557
- PMCID: PMC2882554
- DOI: 10.1136/bmj.c2756
Efficacy of standardised manual therapy and home exercise programme for chronic rotator cuff disease: randomised placebo controlled trial
Abstract
Objective: To investigate the efficacy of a programme of manual therapy and exercise treatment compared with placebo treatment delivered by physiotherapists for people with chronic rotator cuff disease.
Design: Randomised, participant and single assessor blinded, placebo controlled trial.
Setting: Metropolitan region of Melbourne, Victoria, Australia.
Participants: 120 participants with chronic (>3 months) rotator cuff disease recruited through medical practitioners and from the community.
Interventions: The active treatment comprised a manual therapy and home exercise programme; the placebo treatment comprised inactive ultrasound therapy and application of an inert gel. Participants in both groups received 10 sessions of individual standardised treatment over 10 weeks. For the following 12 weeks, the active group continued the home exercise programme and the placebo group received no treatment.
Main outcome measures: The primary outcomes were pain and function measured by the shoulder pain and disability index, average pain on movement measured on an 11 point numerical rating scale, and participants' perceived global rating of overall change.
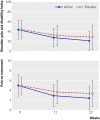
Results: 112 (93%) participants completed the 22 week trial. At 11 weeks no difference was found between groups for change in shoulder pain and disability index (3.6, 95% confidence interval -2.1 to 9.4) or change in pain (0.7, -0.1 to 1.5); both groups showed significant improvements. More participants in the active group reported a successful outcome (defined as "much better"), although the difference was not statistically significant: 42% (24/57) of active participants and 30% (18/61) of placebo participants (relative risk 1.43, 0.87 to 2.34). The active group showed a significantly greater improvement in shoulder pain and disability index than did the placebo group at 22 weeks (between group difference 7.1, 0.3 to 13.9), although no significant difference existed between groups for change in pain (0.9, -0.03 to 1.7) or for the percentage of participants reporting a successful treatment outcome (relative risk 1.39, 0.94 to 2.03). Several secondary outcomes favoured the active group, including shoulder pain and disability index function score, muscle strength, interference with activity, and quality of life.
Conclusion: A standardised programme of manual therapy and home exercise did not confer additional immediate benefits for pain and function compared with a realistic placebo treatment that controlled for therapists' contact in middle aged to older adults with chronic rotator cuff disease. However, greater improvements were apparent at follow-up, particularly in shoulder function and strength, suggesting that benefits with active treatment take longer to manifest.
Trial registration: Clinical trials NCT00415441.
Conflict of interest statement
Competing interests: All authors have completed the Unified Competing Interest form at
Figures
Comment in
-
Manual therapy and home exercise for chronic rotator cuff disease.Clin J Sport Med. 2011 Sep;21(5):464-6. doi: 10.1097/01.JSM.0000405007.94831.06. Clin J Sport Med. 2011. PMID: 21892024 No abstract available.
References
-
- Chard MD, Hazleman R, Hazleman BL, King RH, Reiss BB. Shoulder disorders in the elderly: a community survey. Arthritis Rheum 1991;34:766-9. - PubMed
-
- Roquelaure Y, Ha C, Leclerc A, Touranchet A, Sauteron M, Melchior M, et al. Epidemiologic surveillance of upper-extremity musculoskeletal disorders in the working population. Arthritis Rheum 2006;55:765-78. - PubMed
-
- Smith KL, Harryman DT 2nd, Antoniou J, Campbell B, Sidles JA, Matsen FA 3rd. A prospective, multipractice study of shoulder function and health status in patients with documented rotator cuff tears. J Shoulder Elbow Surg 2000;9:395-402. - PubMed
-
- Bridges-Webb C, Britt H, Miles D, Neary S, Charles J. Morbidity and treatment in general practice in Australia 1990-91. Med J Aust 1992;157:1-56S. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical