Protein analysis of purified respiratory syncytial virus particles reveals an important role for heat shock protein 90 in virus particle assembly
- PMID: 20530633
- PMCID: PMC2938102
- DOI: 10.1074/mcp.M110.001651
Protein analysis of purified respiratory syncytial virus particles reveals an important role for heat shock protein 90 in virus particle assembly
Abstract
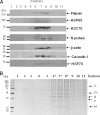
In this study, we used imaging and proteomics to identify the presence of virus-associated cellular proteins that may play a role in respiratory syncytial virus (RSV) maturation. Fluorescence microscopy of virus-infected cells revealed the presence of virus-induced cytoplasmic inclusion bodies and mature virus particles, the latter appearing as virus filaments. In situ electron tomography suggested that the virus filaments were complex structures that were able to package multiple copies of the virus genome. The virus particles were purified, and the protein content was analyzed by one-dimensional nano-LC MS/MS. In addition to all the major virus structural proteins, 25 cellular proteins were also detected, including proteins associated with the cortical actin network, energy pathways, and heat shock proteins (HSP70, HSC70, and HSP90). Representative actin-associated proteins, HSC70, and HSP90 were selected for further biological validation. The presence of beta-actin, filamin-1, cofilin-1, HSC70, and HSP90 in the virus preparation was confirmed by immunoblotting using relevant antibodies. Immunofluorescence microscopy of infected cells stained with antibodies against relevant virus and cellular proteins confirmed the presence of these cellular proteins in the virus filaments and inclusion bodies. The relevance of HSP90 to virus infection was examined using the specific inhibitors 17-N-Allylamino-17-demethoxygeldanamycin. Although virus protein expression was largely unaffected by these drugs, we noted that the formation of virus particles was inhibited, and virus transmission was impaired, suggesting an important role for HSP90 in virus maturation. This study highlights the utility of proteomics in facilitating both our understanding of the role that cellular proteins play during RSV maturation and, by extrapolation, the identification of new potential targets for antiviral therapy.
Figures










References
-
- Grosfeld H., Hill M. G., Collins P. L. (1995) RNA replication by respiratory syncytial virus (RSV) is directed by the N, P, and L proteins; transcription also occurs under these conditions but requires RSV superinfection for efficient synthesis of full-length mRNA. J. Virol. 69, 5677–5686 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

