Neurotoxic effects of TDP-43 overexpression in C. elegans
- PMID: 20530643
- PMCID: PMC2908471
- DOI: 10.1093/hmg/ddq230
Neurotoxic effects of TDP-43 overexpression in C. elegans
Abstract
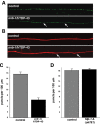
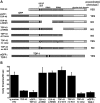
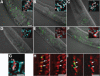
RNA-binding protein TDP-43 has been associated with multiple neurodegenerative diseases, including amyotrophic lateral sclerosis and frontotemporal lobar dementia. We have engineered pan-neuronal expression of human TDP-43 protein in Caenorhabditis elegans, with the goal of generating a convenient in vivo model of TDP-43 function and neurotoxicity. Transgenic worms with the neuronal expression of human TDP-43 exhibit an 'uncoordinated' phenotype and have abnormal motorneuron synapses. Caenorhabditis elegans contains a single putative ortholog of TDP-43, designated TDP-1, which we show can support alternative splicing of CFTR in a cell-based assay. Neuronal overexpression of TDP-1 also results in an uncoordinated phenotype, while genetic deletion of the tdp-1 gene does not affect movement or alter motorneuron synapses. By using the uncoordinated phenotype as a read-out of TDP-43 overexpression neurotoxicty, we have investigated the contribution of specific TDP-43 domains and subcellular localization to toxicity. Full-length (wild-type) human TDP-43 expressed in C. elegans is localized to the nucleus. Deletion of either RNA recognition domain (RRM1 or RRM2) completely blocks neurotoxicity, as does deletion of the C-terminal region. These deleted TDP-43 variants still accumulate in the nucleus, although their subnuclear distribution is altered. Interestingly, fusion of TDP-1 C-terminal sequences to TDP-43 missing its C-terminal domain restores normal subnuclear localization and toxicity in C. elegans and CFTR splicing in cell-based assays. Overexpression of wild-type, full-length TDP-43 in mammalian cells (differentiated M17 cells) can also result in cell toxicity. Our results demonstrate that in vivo TDP-43 neurotoxicity can result from nuclear activity of overexpressed full-length protein.
Figures
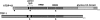



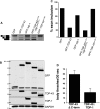
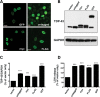
References
-
- Buratti E., Baralle F.E. Characterization and functional implications of the RNA binding properties of nuclear factor TDP-43, a novel splicing regulator of CFTR exon 9. J. Biol. Chem. 2001;276:36337–36343. doi:10.1074/jbc.M104236200. - DOI - PubMed
-
- Strong M.J., Volkening K., Hammond R., Yang W., Strong W., Leystra-Lantz C., Shoesmith C. TDP43 is a human low molecular weight neurofilament (hNFL) mRNA-binding protein. Mol. Cell Neurosci. 2007;35:320–327. doi:10.1016/j.mcn.2007.03.007. - DOI - PubMed
-
- Arai T., Hasegawa M., Akiyama H., Ikeda K., Nonaka T., Mori H., Mann D., Tsuchiya K., Yoshida M., Hashizume Y., Oda T. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 2006;351:602–611. doi:10.1016/j.bbrc.2006.10.093. - DOI - PubMed
-
- Neumann M., Sampathu D.M., Kwong L.K., Truax A.C., Micsenyi M.C., Chou T.T., Bruce J., Schuck T., Grossman M., Clark C.M., et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi:10.1126/science.1134108. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

