Halogenated benzimidazole carboxamides target integrin alpha4beta1 on T-cell and B-cell lymphomas
- PMID: 20530664
- PMCID: PMC3166240
- DOI: 10.1158/0008-5472.CAN-09-3736
Halogenated benzimidazole carboxamides target integrin alpha4beta1 on T-cell and B-cell lymphomas
Abstract
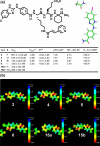
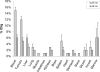
Integrin alpha(4)beta(1) is an attractive but poorly understood target for selective diagnosis and treatment of T-cell and B-cell lymphomas. This report focuses on the rapid microwave preparation, structure-activity relationships, and biological evaluation of medicinally pertinent benzimidazole heterocycles as integrin alpha(4)beta(1) antagonists. We documented tumor uptake of derivatives labeled with (125)I in xenograft murine models of B-cell lymphoma. Molecular homology models of integrin alpha(4)beta(1) predicted that docked halobenzimidazole carboxamides have the halogen atom in a suitable orientation for halogen-hydrogen bonding. The high-affinity halogenated ligands identified offer attractive tools for medicinal and biological use, including fluoro and iodo derivatives with potential radiodiagnostic ((18)F) or radiotherapeutic ((131)I) applications, or chloro and bromo analogues that could provide structural insights into integrin-ligand interactions through photoaffinity, cross-linking/mass spectroscopy, and X-ray crystallographic studies.
Copyright 2010 AACR.
Figures

Similar articles
-
Selectively targeting T- and B-cell lymphomas: a benzothiazole antagonist of alpha4beta1 integrin.J Med Chem. 2009 Jan 8;52(1):14-9. doi: 10.1021/jm800313f. J Med Chem. 2009. PMID: 19072684 Free PMC article.
-
Highly potent, water soluble benzimidazole antagonist for activated alpha 4 beta 1 integrin.J Med Chem. 2007 Nov 15;50(23):5863-7. doi: 10.1021/jm070790o. Epub 2007 Oct 19. J Med Chem. 2007. PMID: 17948981
-
Integrin-specific hydrogels as adaptable tumor organoids for malignant B and T cells.Biomaterials. 2015 Dec;73:110-9. doi: 10.1016/j.biomaterials.2015.09.007. Epub 2015 Sep 11. Biomaterials. 2015. PMID: 26406451 Free PMC article.
-
Design, synthesis, and biological evaluation of radioiodinated benzo[d]imidazole-quinoline derivatives for platelet-derived growth factor receptor β (PDGFRβ) imaging.Bioorg Med Chem. 2019 Jan 15;27(2):383-393. doi: 10.1016/j.bmc.2018.12.016. Epub 2018 Dec 11. Bioorg Med Chem. 2019. PMID: 30563725
-
New drugs for aggressive B-cell and T-cell lymphomas.Lancet Oncol. 2010 Nov;11(11):1074-85. doi: 10.1016/S1470-2045(10)70210-2. Lancet Oncol. 2010. PMID: 21051020 Review.
Cited by
-
A rapid and efficient route to benzazole heterocycles.Nat Protoc. 2010 Nov;5(11):1731-6. doi: 10.1038/nprot.2010.132. Epub 2010 Oct 7. Nat Protoc. 2010. PMID: 21030949
-
Halogen bonding (X-bonding): a biological perspective.Protein Sci. 2013 Feb;22(2):139-52. doi: 10.1002/pro.2201. Epub 2012 Dec 29. Protein Sci. 2013. PMID: 23225628 Free PMC article. Review.
-
The interaction of CCl4 with Ng (Ng = He, Ne, Ar), O2, D2O and ND3: rovibrational energies, spectroscopic constants and theoretical calculations.J Mol Model. 2017 Mar;23(3):87. doi: 10.1007/s00894-017-3269-0. Epub 2017 Feb 21. J Mol Model. 2017. PMID: 28224331
-
From Deworming to Cancer Therapy: Benzimidazoles in Hematological Malignancies.Cancers (Basel). 2024 Oct 12;16(20):3454. doi: 10.3390/cancers16203454. Cancers (Basel). 2024. PMID: 39456548 Free PMC article. Review.
References
-
- Hait WN. Targeted cancer therapeutics. Cancer Res. 2009;69:1263–7. - PubMed
-
- Shimaoka M, Takagi J, Springer TA. Conformational regulation of integrin structure and function. Annu Rev Biophys Biomol Struct. 2002;31:485–516. - PubMed
-
- Peng L, Liu R, Marik J, Wang X, Takada Y, Lam KS. Combinatorial chemistry identifies high-affinity peptidomimetics against α4β1 integrin for in vivo tumor imaging. Nat Chem Biol. 2006;2:381–9. - PubMed
-
- Martin KH, Slack JK, Boerner SA, Martin CC, Parsons T. Integrin connections map: To infinity and beyond. Science. 2002;296:1652–3. - PubMed
-
- Yusuf-Makagiansar H, Anderson ME, Yakovleva TV, Murray JS, Siahaan TJ. Inhibition of LFA-1/ICAM-1 and VLA-4/VCAM-1 as a therapeutic approach to inflammation and autoimmune diseases. Med Res Rev. 2002;22:146–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

