Targeting Notch to target cancer stem cells
- PMID: 20530696
- PMCID: PMC3008160
- DOI: 10.1158/1078-0432.CCR-09-2823
Targeting Notch to target cancer stem cells
Abstract
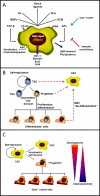
The cellular heterogeneity of neoplasms has been at the center of considerable interest since the "cancer stem cell hypothesis", originally formulated for hematologic malignancies, was extended to solid tumors. The origins of cancer "stem" cells (CSC) or tumor-initiating cells (TIC; henceforth referred to as CSCs) and the methods to identify them are hotly debated topics. Nevertheless, the existence of subpopulations of tumor cells with stem-like characteristics has significant therapeutic implications. The stem-like phenotype includes indefinite self-replication, pluripotency, and, importantly, resistance to chemotherapeutics. Thus, it is plausible that CSCs, regardless of their origin, may escape standard therapies and cause disease recurrences and/or metastasis after apparently complete remissions. Consequently, the idea of selectively targeting CSCs with novel therapeutics is gaining considerable interest. The Notch pathway is one of the most intensively studied putative therapeutic targets in CSC, and several investigational Notch inhibitors are being developed. However, successful targeting of Notch signaling in CSC will require a thorough understanding of Notch regulation and the context-dependent interactions between Notch and other therapeutically relevant pathways. Understanding these interactions will increase our ability to design rational combination regimens that are more likely to prove safe and effective. Additionally, to determine which patients are most likely to benefit from treatment with Notch-targeting therapeutics, reliable biomarkers to measure pathway activity in CSC from specific tumors will have to be identified and validated. This article summarizes the most recent developments in the field of Notch-targeted cancer therapeutics, with emphasis on CSC.
(c) 2010 AACR.
Figures


References
-
- Al Hajj M, Becker MW, Wicha M, Weissman I, Clarke MF. Therapeutic implications of cancer stem cells. Curr Opin Genet Dev. 2004;14:43–7. - PubMed
-
- Donnenberg VS, Donnenberg AD. Multiple drug resistance in cancer revisited: the cancer stem cell hypothesis. J Clin Pharmacol. 2005;45:872–7. - PubMed
-
- Haraguchi N, Inoue H, Tanaka F, et al. Cancer stem cells in human gastrointestinal cancers. Hum Cell. 2006;19:24–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

