The mechanism of ascorbic acid-induced differentiation of ATDC5 chondrogenic cells
- PMID: 20530736
- PMCID: PMC2928517
- DOI: 10.1152/ajpendo.00145.2010
The mechanism of ascorbic acid-induced differentiation of ATDC5 chondrogenic cells
Abstract
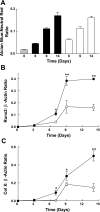
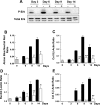
The ATDC5 cell line exhibits a multistep process of chondrogenic differentiation analogous to that observed during endochondral bone formation. Previous investigators have induced ATDC5 cells to differentiate by exposing them to insulin at high concentrations. We have observed spontaneous differentiation of ATDC5 cells maintained in ascorbic acid-containing alpha-MEM. A comparison of the differentiation events in response to high-dose insulin vs. ascorbic acid showed similar expression patterns of key genes, including collagen II, Runx2, Sox9, Indian hedgehog, and collagen X. We took advantage of the action of ascorbic acid to examine signaling events associated with differentiation. In contrast to high-dose insulin, which downregulates both IGF-I and insulin receptors, there were only minimal changes in the abundance of these receptors during ascorbic acid-induced differentiation. Furthermore, ascorbic acid exposure was associated with ERK activation, and ERK inhibition attenuated ascorbic acid-induced differentiation. This was in contrast to the inhibitory effect of ERK activation during IGF-I-induced differentiation. Inhibition of collagen formation with a proline analog markedly attenuated the differentiating effect of ascorbic acid on ATDC5 cells. When plates were conditioned with ATDC5 cells exposed to ascorbic acid, ATDC5 cells were able to differentiate in the absence of ascorbic acid. Our results indicate that matrix formation early in the differentiation process is essential for ascorbic acid-induced ATDC5 differentiation. We conclude that ascorbic acid can promote the differentiation of ATDC5 cells by promoting the formation of collagenous matrix and that matrix formation mediates activation of the ERK signaling pathway, which promotes the differentiation program.
Figures







Similar articles
-
Insulin-like growth factor-I signaling is modified during chondrocyte differentiation.J Endocrinol. 2004 Dec;183(3):477-86. doi: 10.1677/joe.1.05873. J Endocrinol. 2004. PMID: 15590974
-
Ascorbic acid enhances chondrocyte differentiation of ATDC5 by accelerating insulin receptor signaling.Cell Biol Int. 2023 Oct;47(10):1737-1748. doi: 10.1002/cbin.12067. Epub 2023 Jun 28. Cell Biol Int. 2023. PMID: 37381608
-
Sequential progression of the differentiation program by bone morphogenetic protein-2 in chondrogenic cell line ATDC5.Exp Cell Res. 1998 May 25;241(1):1-11. doi: 10.1006/excr.1998.4045. Exp Cell Res. 1998. PMID: 9633508
-
The role of insulin in chondrogenesis.Mol Cell Endocrinol. 2006 Apr 25;249(1-2):107-15. doi: 10.1016/j.mce.2006.02.002. Epub 2006 Mar 13. Mol Cell Endocrinol. 2006. PMID: 16530934
-
p21(Cip-1/SDI-1/WAF-1) expression via the mitogen-activated protein kinase signaling pathway in insulin-induced chondrogenic differentiation of ATDC5 cells.Biochem Biophys Res Commun. 2004 Aug 6;320(4):1069-75. doi: 10.1016/j.bbrc.2004.06.057. Biochem Biophys Res Commun. 2004. PMID: 15249198
Cited by
-
Proteome analysis of human mesenchymal stem cells undergoing chondrogenesis when exposed to the products of various magnesium-based materials degradation.Bioact Mater. 2019 Apr 24;4:168-188. doi: 10.1016/j.bioactmat.2019.04.001. eCollection 2019 Dec. Bioact Mater. 2019. PMID: 31049466 Free PMC article.
-
Aggrecan is required for chondrocyte differentiation in ATDC5 chondroprogenitor cells.PLoS One. 2019 Jun 17;14(6):e0218399. doi: 10.1371/journal.pone.0218399. eCollection 2019. PLoS One. 2019. PMID: 31206541 Free PMC article.
-
Development of Anti-OSCAR Antibodies for the Treatment of Osteoarthritis.Biomedicines. 2023 Oct 19;11(10):2844. doi: 10.3390/biomedicines11102844. Biomedicines. 2023. PMID: 37893216 Free PMC article.
-
Functional O-GlcNAc modifications: implications in molecular regulation and pathophysiology.Crit Rev Biochem Mol Biol. 2014 Mar-Apr;49(2):140-163. doi: 10.3109/10409238.2014.884535. Epub 2014 Feb 14. Crit Rev Biochem Mol Biol. 2014. PMID: 24524620 Free PMC article. Review.
-
Synergistic effect of ascorbic acid and collagen addition on the increase in type 2 collagen accumulation in cartilage-like MSC sheet.Cytotechnology. 2017 Jun;69(3):405-416. doi: 10.1007/s10616-015-9924-3. Epub 2015 Nov 16. Cytotechnology. 2017. PMID: 26572654 Free PMC article.
References
-
- Adams JC, Watt FM. Regulation of development and differentiation by the extracellular matrix. Development 117: 1183–1198, 1993 - PubMed
-
- Altaf FM, Hering TM, Kazmi NH, Yoo JU, Johnstone B. Ascorbate-enhanced chondrogenesis of ATDC5 cells. Eur Cell Mater 12: 64–69, 2006 - PubMed
-
- Aronow MA, Gerstenfeld LC, Owen TA, Tassinari MS, Stein GS, Lian JB. Factors that promote progressive development of the osteoblast phenotype in cultured fetal rat calvaria cells. J Cell Physiol 143: 213–221, 1990 - PubMed
-
- Atsumi T, Miwa Y, Kimata K, Ikawa Y. A chondrogenic cell line derived from a differentiating culture of AT805 teratocarcinoma cells. Cell Differ Dev 30: 109–116, 1990 - PubMed
-
- Bosnakovski D, Mizuno M, Kim G, Takagi S, Okumura M, Fujinaga T. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnol Bioeng 93: 1152–1163, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

