A chemotactic gradient sequestered on endothelial heparan sulfate induces directional intraluminal crawling of neutrophils
- PMID: 20530797
- PMCID: PMC3173988
- DOI: 10.1182/blood-2010-01-266072
A chemotactic gradient sequestered on endothelial heparan sulfate induces directional intraluminal crawling of neutrophils
Abstract
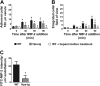
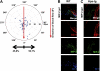
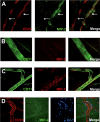
During infection, chemokines sequestered on endothelium induce recruitment of circulating leukocytes into the tissue where they chemotax along chemokine gradients toward the afflicted site. The aim of this in vivo study was to determine whether a chemokine gradient was formed intravascularly and influenced intraluminal neutrophil crawling and transmigration. A chemokine gradient was induced by placing a macrophage inflammatory protein-2 (MIP-2)-containing (CXCL2) gel on the cremaster muscle of anesthetized wild-type mice or heparanase-overexpressing transgenic mice (hpa-tg) with truncated heparan sulfate (HS) side chains. Neutrophil-endothelial interactions were visualized by intravital microscopy and chemokine gradients detected by confocal microscopy. Localized extravascular chemokine release (MIP-2 gel) induced directed neutrophil crawling along a chemotactic gradient immobilized on the endothelium and accelerated their recruitment into the target tissue compared with homogeneous extravascular chemokine concentration (MIP-2 superfusion). Endothelial chemokine sequestration occurred exclusively in venules and was HS-dependent, and neutrophils in hpa-tg mice exhibited random crawling. Despite similar numbers of adherent neutrophils in hpa-tg and wild-type mice, the altered crawling in hpa-tg mice was translated into decreased number of emigrated neutrophils and ultimately decreased the ability to clear bacterial infections. In conclusion, an intravascular chemokine gradient sequestered by endothelial HS effectively directs crawling leukocytes toward transmigration loci close to the infection site.
Figures

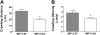

Similar articles
-
Tracking neutrophil intraluminal crawling, transendothelial migration and chemotaxis in tissue by intravital video microscopy.J Vis Exp. 2011 Sep 24;(55):3296. doi: 10.3791/3296. J Vis Exp. 2011. PMID: 21968530 Free PMC article.
-
CXC chemokines, MIP-2 and KC, induce P-selectin-dependent neutrophil rolling and extravascular migration in vivo.Br J Pharmacol. 2001 Jun;133(3):413-21. doi: 10.1038/sj.bjp.0704087. Br J Pharmacol. 2001. PMID: 11375258 Free PMC article.
-
Pharmacological inhibition of p38 mitogen-activated protein kinases affects KC/CXCL1-induced intraluminal crawling, transendothelial migration, and chemotaxis of neutrophils in vivo.Mediators Inflamm. 2013;2013:290565. doi: 10.1155/2013/290565. Epub 2013 Mar 4. Mediators Inflamm. 2013. PMID: 23533303 Free PMC article.
-
Heparan sulphate as a regulator of leukocyte recruitment in inflammation.Curr Protein Pept Sci. 2015;16(1):77-86. doi: 10.2174/1573402111666150213165054. Curr Protein Pept Sci. 2015. PMID: 25692849 Review.
-
Tissue-specific neutrophil recruitment into the lung, liver, and kidney.J Innate Immun. 2013;5(4):348-57. doi: 10.1159/000345943. Epub 2012 Dec 21. J Innate Immun. 2013. PMID: 23257511 Free PMC article. Review.
Cited by
-
Monosodium urate crystals induce extracellular DNA traps in neutrophils, eosinophils, and basophils but not in mononuclear cells.Front Immunol. 2012 Sep 3;3:277. doi: 10.3389/fimmu.2012.00277. eCollection 2012. Front Immunol. 2012. PMID: 22969769 Free PMC article.
-
Impact of heparanase and the tumor microenvironment on cancer metastasis and angiogenesis: basic aspects and clinical applications.Rambam Maimonides Med J. 2011 Jan 31;2(1):e0019. doi: 10.5041/RMMJ.10019. Print 2011 Jan. Rambam Maimonides Med J. 2011. PMID: 23908791 Free PMC article.
-
Heparanase overexpression impairs inflammatory response and macrophage-mediated clearance of amyloid-β in murine brain.Acta Neuropathol. 2012 Oct;124(4):465-78. doi: 10.1007/s00401-012-0997-1. Epub 2012 Jun 13. Acta Neuropathol. 2012. PMID: 22692572 Free PMC article.
-
Differential Effects of Posttranslational Modifications of CXCL8/Interleukin-8 on CXCR1 and CXCR2 Internalization and Signaling Properties.Int J Mol Sci. 2018 Nov 27;19(12):3768. doi: 10.3390/ijms19123768. Int J Mol Sci. 2018. PMID: 30486423 Free PMC article.
-
Overview of the Mechanisms that May Contribute to the Non-Redundant Activities of Interferon-Inducible CXC Chemokine Receptor 3 Ligands.Front Immunol. 2018 Jan 15;8:1970. doi: 10.3389/fimmu.2017.01970. eCollection 2017. Front Immunol. 2018. PMID: 29379506 Free PMC article. Review.
References
-
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7(9):678–689. - PubMed
-
- Petri B, Phillipson M, Kubes P. The physiology of leukocyte recruitment: an in vivo perspective. J Immunol. 2008;180(10):6439–6446. - PubMed
-
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76(2):301–314. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

