2-Deoxy-D-glucose regulates dedifferentiation through beta-catenin pathway in rabbit articular chondrocytes
- PMID: 20530983
- PMCID: PMC2912477
- DOI: 10.3858/emm.2010.42.7.051
2-Deoxy-D-glucose regulates dedifferentiation through beta-catenin pathway in rabbit articular chondrocytes
Abstract
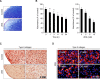
2-deoxy-D-glucose (2DG) is known as a synthetic inhibitor of glucose. 2DG regulates various cellular responses including proliferation, apoptosis and differentiation by regulation of glucose metabolism in cancer cells. However, the effects of 2DG in normal cells, including chondrocytes, are not clear yet. We examined the effects of 2DG on dedifferentiation with a focus on the beta-catenin pathway in rabbit articular chondrocytes. The rabbit articular chondrocytes were treated with 5 mM 2DG for the indicated time periods or with various concentrations of 2DG for 24 h, and the expression of type II collagen, c-jun and beta-catenin was determined by Western blot, RT-PCR, immunofluorescence staining and immunohistochemical staining and reduction of sulfated proteoglycan synthesis detected by Alcain blue staining. Luciferase assay using a TCF (T cell factor)/LEF (lymphoid enhancer factor) reporter construct was used to demonstrate the transcriptional activity of beta-catenin. We found that 2DG treatment caused a decrease of type II collagen expression. 2DG induced dedifferentiation was dependent on activation of beta-catenin, as the 2DG stimulated accumulation of beta-catenin, which is characterized by translocation of beta-catenin into the nucleus determined by immunofluorescence staining and luciferase assay. Inhibition of beta-catenin degradation by inhibition of glycogen synthase kinase 3-beta with lithium chloride (LiCl) or inhibition of proteasome with z-Leu-Leu-Leu-CHO (MG132) accelerated the decrease of type II collagen expression in the chondrocytes. 2DG regulated the post-translational level of beta-catenin whereas the transcriptional level of beta-catenin was not altered. These results collectively showed that 2DG regulates dedifferentiation via beta-catenin pathway in rabbit articular chondrocytes.
Figures



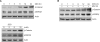
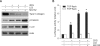


References
-
- Blom AB, Brockbank SM, van Lent PL, van Beuningen HM, Geurts J, Takahashi N, van der Kraan PM, van de Loo FA, Schreurs BW, Clements K, Newham P, van den Berg WB. Involvement of the Wnt signaling pathway in experimental and human osteoarthritis: prominent role of Wnt-induced signaling protein 1. Arthritis Rheum. 2009;60:501–512. - PubMed
-
- Charni-Ben Tabassi N, Garnero P. Monitoring cartilage turnover. Curr Rheumatol Rep. 2007;9:16–24. - PubMed
-
- Cho SH, Oh CD, Kim SJ, Kim IC, Chun JS. Retinoic acid inhibits chondrogenesis of mesenchymal cells by sustaining expression of N-cadherin and its associated proteins. J Cell Biochem. 2003;89:837–847. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
