Intestinal FXR-mediated FGF15 production contributes to diurnal control of hepatic bile acid synthesis in mice
- PMID: 20531290
- PMCID: PMC6643294
- DOI: 10.1038/labinvest.2010.107
Intestinal FXR-mediated FGF15 production contributes to diurnal control of hepatic bile acid synthesis in mice
Abstract
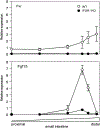
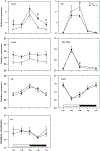
Hepatic bile acid synthesis is subject to complex modes of transcriptional control, in which the bile acid-activated nuclear receptor farnesoid X receptor (FXR) in liver and intestine-derived, FXR-controlled fibroblast growth factor 15 (Fgf15) are involved. The Fgf15 pathway is assumed to contribute significantly to control of hepatic bile acid synthesis. However, scientific evidence supporting this assumption is primarily based on gene expression data. Using intestine-selective FXR knockout mice (iFXR-KO), we show that contribution of intestinal FXR-Fgf15 signalling in regulation of hepatic cholesterol 7α-hydroxylase (Cyp7A1) expression depends on time of the day with increased hepatic Cyp7A1 expression in iFXR-KO mice compared with controls exclusively during the dark phase. To assess the physiological relevance hereof, we determined effects of intestine-selective deletion of FXR on physiological parameters such as bile formation and kinetics of the enterohepatic circulation of bile acids. It appeared that intestinal FXR deficiency leads to a modest but significant increase in cholic acid pool size, without changes in fractional turnover rate. As a consequence, bile flow and biliary bile acid secretion rates were increased in iFXR-KO mice compared with controls. Feeding a bile acid-containing diet or treatment with a bile acid sequestrant similarly affected bile formation in iFXR-KO and control mice and induced similar changes in Cyp7A1 and Cyp8B1 expression patterns. In conclusion, this study is the first to demonstrate the physiological relevance of the contribution of the intestinal FXR-Fgf15 signalling pathway in control of hepatic bile acid synthesis. Fgf15 contributes to the regulation of hepatic bile acid synthesis in mice mainly during the dark phase. Expansion of the circulating bile acid pool as well as bile acid sequestration diminishes the contribution of intestinal FXR-Fgf15 signalling in control of hepatic bile acid synthesis and bile formation.
Conflict of interest statement
DISCLOSURE/CONFLICT OF INTEREST
The authors declare no conflict of interest.
Figures





Similar articles
-
Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine.J Lipid Res. 2007 Dec;48(12):2664-72. doi: 10.1194/jlr.M700330-JLR200. Epub 2007 Aug 24. J Lipid Res. 2007. PMID: 17720959
-
Enterohepatic circulation of bile salts in farnesoid X receptor-deficient mice: efficient intestinal bile salt absorption in the absence of ileal bile acid-binding protein.J Biol Chem. 2003 Oct 24;278(43):41930-7. doi: 10.1074/jbc.M306309200. Epub 2003 Aug 12. J Biol Chem. 2003. PMID: 12917447
-
Protective effects of farnesoid X receptor (FXR) on hepatic lipid accumulation are mediated by hepatic FXR and independent of intestinal FGF15 signal.Liver Int. 2015 Apr;35(4):1133-1144. doi: 10.1111/liv.12456. Epub 2014 Feb 7. Liver Int. 2015. PMID: 25156247 Free PMC article.
-
Bile Acids as Hormones: The FXR-FGF15/19 Pathway.Dig Dis. 2015;33(3):327-31. doi: 10.1159/000371670. Epub 2015 May 27. Dig Dis. 2015. PMID: 26045265 Free PMC article. Review.
-
Bile acids: regulation of synthesis.J Lipid Res. 2009 Oct;50(10):1955-66. doi: 10.1194/jlr.R900010-JLR200. Epub 2009 Apr 3. J Lipid Res. 2009. PMID: 19346330 Free PMC article. Review.
Cited by
-
All-trans retinoic acid regulates hepatic bile acid homeostasis.Biochem Pharmacol. 2014 Oct 15;91(4):483-9. doi: 10.1016/j.bcp.2014.08.018. Epub 2014 Aug 28. Biochem Pharmacol. 2014. PMID: 25175738 Free PMC article.
-
High-fat diet promotes gestational diabetes mellitus through modulating gut microbiota and bile acid metabolism.Front Microbiol. 2025 Jan 28;15:1480446. doi: 10.3389/fmicb.2024.1480446. eCollection 2024. Front Microbiol. 2025. PMID: 39935515 Free PMC article.
-
FGF/FGFR signaling in health and disease.Signal Transduct Target Ther. 2020 Sep 2;5(1):181. doi: 10.1038/s41392-020-00222-7. Signal Transduct Target Ther. 2020. PMID: 32879300 Free PMC article. Review.
-
Biological tuners to reshape the bile acid pool for therapeutic purposes in non-alcoholic fatty liver disease.Clin Sci (Lond). 2023 Jan 13;137(1):65-85. doi: 10.1042/CS20220697. Clin Sci (Lond). 2023. PMID: 36601783 Free PMC article. Review.
-
Lowering bile acid pool size with a synthetic farnesoid X receptor (FXR) agonist induces obesity and diabetes through reduced energy expenditure.J Biol Chem. 2011 Jul 29;286(30):26913-20. doi: 10.1074/jbc.M111.248203. Epub 2011 Jun 1. J Biol Chem. 2011. PMID: 21632533 Free PMC article.
References
-
- Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem 2003;72:137–174. - PubMed
-
- Cai SY, Boyer JL. FXR: a target for cholestatic syndromes? Expert Opin Ther Targets 2006;10:409–421. - PubMed
-
- Kok T, Hulzebos CV, Wolters H, et al. Enterohepatic circulation of bile salts in farnesoid X receptor-deficient mice: efficient intestinal bile salt absorption in the absence of ileal bile acid-binding protein. J Biol Chem 2003;278:41930–41937. - PubMed
-
- Sinal CJ, Tohkin M, Miyata M, et al. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 2000;102:731–744. - PubMed
-
- Forman BM, Goode E, Chen J, et al. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell 1995;81:687–693. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

