Emerging role of Lys-63 ubiquitination in protein kinase and phosphatase activation and cancer development
- PMID: 20531303
- PMCID: PMC3008764
- DOI: 10.1038/onc.2010.190
Emerging role of Lys-63 ubiquitination in protein kinase and phosphatase activation and cancer development
Abstract
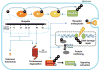
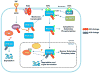
Ubiquitination is an important post-translational modification that has a pivotal role in numerous biological functions, such as cell growth, proliferation, apoptosis, DNA damage response, innate immune response and neuron degeneration. Although ubiquitination is thought to achieve these functions by targeting proteins for proteasome-dependent degradation, recent studies suggest that ubiquitination also has nonproteolytic functions, such as protein trafficking, kinase and phosphatase activation, which are involved in cell survival and cancer development. These progresses have advanced our current understanding of the novel functions of ubiquitination in signal transduction pathways and may provide novel paradigms for the treatment of human cancers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
K63-linked ubiquitination in kinase activation and cancer.Front Oncol. 2012 Jan 31;2:5. doi: 10.3389/fonc.2012.00005. eCollection 2012. Front Oncol. 2012. PMID: 22649774 Free PMC article.
-
Crosstalk between kinases, phosphatases and miRNAs in cancer.Biochimie. 2014 Dec;107 Pt B:167-87. doi: 10.1016/j.biochi.2014.09.011. Epub 2014 Sep 16. Biochimie. 2014. PMID: 25230087 Review.
-
Human cancer, PTEN and the PI-3 kinase pathway.Semin Cell Dev Biol. 2004 Apr;15(2):171-6. doi: 10.1016/j.semcdb.2003.12.021. Semin Cell Dev Biol. 2004. PMID: 15209376 Review.
-
How important is the phosphatase activity of sensor kinases?Curr Opin Microbiol. 2010 Apr;13(2):168-76. doi: 10.1016/j.mib.2010.01.013. Epub 2010 Mar 10. Curr Opin Microbiol. 2010. PMID: 20223700 Free PMC article. Review.
-
Illumination of mitotic orchestra during cell division: a Polo view.Cell Signal. 2011 Jan;23(1):1-5. doi: 10.1016/j.cellsig.2010.07.003. Epub 2010 Jul 12. Cell Signal. 2011. PMID: 20633640 Free PMC article. Review.
Cited by
-
E3 ubiquitin ligase NKLAM ubiquitinates STAT1 and positively regulates STAT1-mediated transcriptional activity.Cell Signal. 2016 Dec;28(12):1833-1841. doi: 10.1016/j.cellsig.2016.08.014. Epub 2016 Aug 26. Cell Signal. 2016. PMID: 27570112 Free PMC article.
-
Skp2-macroH2A1-CDK8 axis orchestrates G2/M transition and tumorigenesis.Nat Commun. 2015 Mar 30;6:6641. doi: 10.1038/ncomms7641. Nat Commun. 2015. PMID: 25818643 Free PMC article.
-
miR-130b, an onco-miRNA in bladder cancer, is directly regulated by NF-κB and sustains NF-κB activation by decreasing Cylindromatosis expression.Oncotarget. 2016 Jul 26;7(30):48547-48561. doi: 10.18632/oncotarget.10423. Oncotarget. 2016. PMID: 27391066 Free PMC article.
-
Cellular functions and molecular mechanisms of ubiquitination in osteosarcoma.Front Oncol. 2022 Dec 1;12:1072701. doi: 10.3389/fonc.2022.1072701. eCollection 2022. Front Oncol. 2022. PMID: 36530999 Free PMC article.
-
F-box only protein 31 (FBXO31) negatively regulates p38 mitogen-activated protein kinase (MAPK) signaling by mediating lysine 48-linked ubiquitination and degradation of mitogen-activated protein kinase kinase 6 (MKK6).J Biol Chem. 2014 Aug 1;289(31):21508-18. doi: 10.1074/jbc.M114.560342. Epub 2014 Jun 16. J Biol Chem. 2014. PMID: 24936062 Free PMC article.
References
-
- Adhikari A, Chen ZJ. Dev Cell. 2009;16:485–6. - PubMed
-
- Adhikary S, Eilers M. Nat Rev Mol Cell Biol. 2005;6:635–45. - PubMed
-
- Adhikary S, Marinoni F, Hock A, Hulleman E, Popov N, Beier R, Bernard S, Quarto M, Capra M, Goettig S, Kogel U, Scheffner M, Helin K, Eilers M. Cell. 2005;123:409–21. - PubMed
-
- Amati B, Sanchez-Arevalo Lobo VJ. Nat Cell Biol. 2007;9:729–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

