The NADPH oxidases NOX4 and DUOX2 regulate cell cycle entry via a p53-dependent pathway
- PMID: 20531308
- PMCID: PMC2916958
- DOI: 10.1038/onc.2010.200
The NADPH oxidases NOX4 and DUOX2 regulate cell cycle entry via a p53-dependent pathway
Abstract
Reactive oxygen species (ROS) are produced in growth factor-signaling pathways leading to cell proliferation, but the mechanisms leading to ROS generation and the targets of ROS signals are not well understood. Using a focused siRNA screen to identify redox-related proteins required for growth factor-induced cell cycle entry, we show that two ROS-generating proteins, the NADPH oxidases NOX4 and DUOX2, are required for platelet-derived growth factor (PDGF) induced retinoblastoma protein (Rb) phosphorylation in normal human fibroblasts. Unexpectedly, NOX4 and DUOX2 knockdown did not inhibit the early signaling pathways leading to cyclin D1 upregulation. However, hours after growth factor stimulation, NOX4 and DUOX2 knockdown reduced ERK1 phosphorylation and increased levels of the tumor suppressor protein p53 and a cell cycle inhibitor protein p21 (Waf1/Cip1) that is transcriptionally regulated by p53. Co-knockdown of NOX4 or DUOX2 with either p53 or with p21 overcame the inhibition of Rb phosphorylation that occurred with NOX4 or DUOX2 knockdown alone. Our results argue that rather than primarily affecting growth factor receptor signaling, NOX4 and DUOX2 regulate cell cycle entry as part of a p53-dependent checkpoint for proliferation.
Conflict of interest statement
Conflict of Interest
The authors declare no conflict of interest.
Figures
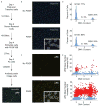

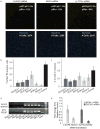
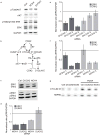
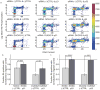

References
-
- Bae YS, Kang SW, Seo MS, Baines IC, Tekle E, Chock PB, et al. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. The Journal of biological chemistry. 1997;272:217–221. - PubMed
-
- Bae YS, Sung JY, Kim OS, Kim YJ, Hur KC, Kazlauskas A, et al. Platelet-derived growth factor-induced H(2)O(2) production requires the activation of phosphatidylinositol 3-kinase. The Journal of biological chemistry. 2000;275:10527–10531. - PubMed
-
- Burch PM, Heintz NH. Redox regulation of cell-cycle re-entry: cyclin D1 as a primary target for the mitogenic effects of reactive oxygen and nitrogen species. Antioxidants & redox signaling. 2005;7:741–751. - PubMed
-
- Burhans WC, Heintz NH. The cell cycle is a redox cycle: Linking phase-specific targets to cell fate. Free radical biology & medicine 2009 - PubMed
-
- Chambard JC, Lefloch R, Pouyssegur J, Lenormand P. ERK implication in cell cycle regulation. Biochimica et biophysica acta. 2007;1773:1299–1310. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

