A new organellar complex in rat sympathetic neurons
- PMID: 20531934
- PMCID: PMC2877718
- DOI: 10.1371/journal.pone.0010872
A new organellar complex in rat sympathetic neurons
Abstract
Membranous compartments of neurons such as axons, dendrites and modified primary cilia are defining features of neuronal phenotype. This is unlike organelles deep to the plasma membrane, which are for the most part generic and not related directly to morphological, neurochemical or functional specializations. However, here we use multi-label immunohistochemistry combined with confocal and electron microscopy to identify a very large (approximately 6 microns in diameter), entirely intracellular neuronal organelle which occurs singly in a ubiquitous but neurochemically distinct and morphologically simple subset of sympathetic ganglion neurons. Although usually toroidal, it also occurs as twists or rods depending on its intracellular position: tori are most often perinuclear whereas rods are often found in axons. These 'loukoumasomes' (doughnut-like bodies) bind a monoclonal antibody raised against beta-III-tubulin (SDL.3D10), although their inability to bind other beta-III-tubulin monoclonal antibodies indicate that the responsible antigen is not known. Position-morphology relationships within neurons and their expression of non-muscle heavy chain myosin suggest a dynamic structure. They associate with nematosomes, enigmatic nucleolus-like organelles present in many neural and non-neural tissues, which we now show to be composed of filamentous actin. Loukoumasomes also separately interact with mother centrioles forming the basal body of primary cilia. They express gamma tubulin, a microtubule nucleator which localizes to non-neuronal centrosomes, and cenexin, a mother centriole-associated protein required for ciliogenesis. These data reveal a hitherto undescribed organelle, and depict it as an intracellular transport machine, shuttling material between the primary cilium, the nematosome, and the axon.
Conflict of interest statement
Figures
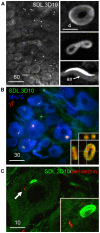
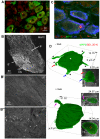
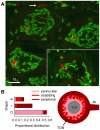
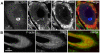
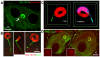
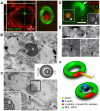
Similar articles
-
Loukoumasomes Are Distinct Subcellular Structures from Rods and Rings and Are Structurally Associated with MAP2 and the Nuclear Envelope in Retinal Cells.PLoS One. 2016 Oct 31;11(10):e0165162. doi: 10.1371/journal.pone.0165162. eCollection 2016. PLoS One. 2016. PMID: 27798680 Free PMC article.
-
Biochemical and immunological analyses of cytoskeletal domains of neurons.J Cell Biol. 1986 Jan;102(1):252-62. doi: 10.1083/jcb.102.1.252. J Cell Biol. 1986. PMID: 3510221 Free PMC article.
-
Vacuole dynamics in growth cones: correlated EM and video observations.J Neurosci. 1993 Aug;13(8):3375-93. doi: 10.1523/JNEUROSCI.13-08-03375.1993. J Neurosci. 1993. PMID: 8340814 Free PMC article.
-
Myosin Va movements in normal and dilute-lethal axons provide support for a dual filament motor complex.J Cell Biol. 1999 Sep 6;146(5):1045-60. doi: 10.1083/jcb.146.5.1045. J Cell Biol. 1999. PMID: 10477758 Free PMC article.
-
The Rise of the Cartwheel: Seeding the Centriole Organelle.Bioessays. 2018 Apr;40(4):e1700241. doi: 10.1002/bies.201700241. Epub 2018 Mar 6. Bioessays. 2018. PMID: 29508910 Review.
Cited by
-
Loukoumasomes Are Distinct Subcellular Structures from Rods and Rings and Are Structurally Associated with MAP2 and the Nuclear Envelope in Retinal Cells.PLoS One. 2016 Oct 31;11(10):e0165162. doi: 10.1371/journal.pone.0165162. eCollection 2016. PLoS One. 2016. PMID: 27798680 Free PMC article.
-
Compositional complexity of rods and rings.Mol Biol Cell. 2018 Sep 15;29(19):2303-2316. doi: 10.1091/mbc.E18-05-0274. Epub 2018 Jul 19. Mol Biol Cell. 2018. PMID: 30024290 Free PMC article.
-
Anti-rods and rings autoantibodies can occur in the hepatitis c-naïve population.J Prev Med Hyg. 2013 Sep;54(3):175-80. J Prev Med Hyg. 2013. PMID: 24783898 Free PMC article.
-
Induction of cytoplasmic rods and rings structures by inhibition of the CTP and GTP synthetic pathway in mammalian cells.PLoS One. 2011;6(12):e29690. doi: 10.1371/journal.pone.0029690. Epub 2011 Dec 29. PLoS One. 2011. PMID: 22220215 Free PMC article.
-
Microtubular and Nuclear Functions of γ-Tubulin: Are They LINCed?Cells. 2019 Mar 19;8(3):259. doi: 10.3390/cells8030259. Cells. 2019. PMID: 30893853 Free PMC article. Review.
References
-
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, et al. New York: Garland Science; 2008. Molecular Biology of the Cell.1392
-
- Ockleford CD, Nevard CH, Indans I, Jones CJ. Structure and function of the nematosome. J Cell Sci. 1987;87 (Pt1):27–44. - PubMed
-
- Li SH, Li H, Torre ER, Li XJ. Expression of huntingtin-associated protein-1 in neuronal cells implicates a role in neuritic growth. Mol Cell Neurosci. 2000;16:168–183. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

