Evidence for a role of endocannabinoids, astrocytes and p38 phosphorylation in the resolution of postoperative pain
- PMID: 20531936
- PMCID: PMC2878341
- DOI: 10.1371/journal.pone.0010891
Evidence for a role of endocannabinoids, astrocytes and p38 phosphorylation in the resolution of postoperative pain
Abstract
Background: An alarming portion of patients develop persistent or chronic pain following surgical procedures, but the mechanisms underlying the transition from acute to chronic pain states are not fully understood. In general, endocannabinoids (ECBs) inhibit nociceptive processing by stimulating cannabinoid receptors type 1 (CB(1)) and type 2 (CB(2)). We have previously shown that intrathecal administration of a CB(2) receptor agonist reverses both surgical incision-induced behavioral hypersensitivity and associated over-expression of spinal glial markers. We therefore hypothesized that endocannabinoid signaling promotes the resolution of acute postoperative pain by modulating pro-inflammatory signaling in spinal cord glial cells.
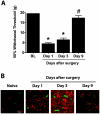
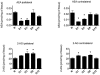
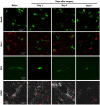
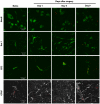
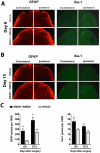
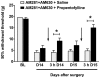
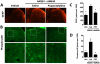
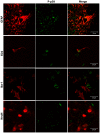
Methodology/principal findings: To test this hypothesis, rats receiving paw incision surgery were used as a model of acute postoperative pain that spontaneously resolves. We first characterized the concentration of ECBs and localization of CB(1) and CB(2) receptors in the spinal cord following paw incision. We then administered concomitant CB(1) and CB(2) receptor antagonists/inverse agonists (AM281 and AM630, 1 mg x kg(-1) each, i.p.) during the acute phase of paw incision-induced mechanical allodynia and evaluated the expression of glial cell markers and phosphorylated p38 (a MAPK associated with inflammation) in the lumbar dorsal horn. Dual blockade of CB(1) and CB(2) receptor signaling prevented the resolution of postoperative allodynia and resulted in persistent over-expression of spinal Glial Fibrillary Acidic Protein (GFAP, an astrocytic marker) and phospho-p38 in astrocytes. We provide evidence for the functional significance of these astrocytic changes by demonstrating that intrathecal administration of propentofylline (50 microg, i.t.) attenuated both persistent behavioral hypersensitivity and over-expression of GFAP and phospho-p38 in antagonist-treated animals.
Conclusions/significance: Our results demonstrate that endocannabinoid signaling via CB(1) and CB(2) receptors is necessary for the resolution of paw incision-induced behavioral hypersensitivity and for the limitation of pro-inflammatory signaling in astrocytes following surgical insult. Our findings suggest that therapeutic strategies designed to enhance endocannabinoid signaling may prevent patients from developing persistent or chronic pain states following surgery.
Conflict of interest statement
Figures
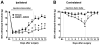
References
-
- Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–1625. - PubMed
-
- Romero-Sandoval A, Eisenach JC. Spinal cannabinoid receptor type 2 activation reduces hypersensitivity and spinal cord glial activation after paw incision. Anesthesiology. 2007;106:787–794. - PubMed
-
- Scott DA, Wright CE, Angus JA. Evidence that CB-1 and CB-2 cannabinoid receptors mediate antinociception in neuropathic pain in the rat. Pain. 2004;109:124–131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

