Adjuvanted influenza vaccine administered intradermally elicits robust long-term immune responses that confer protection from lethal challenge
- PMID: 20531947
- PMCID: PMC2878352
- DOI: 10.1371/journal.pone.0010897
Adjuvanted influenza vaccine administered intradermally elicits robust long-term immune responses that confer protection from lethal challenge
Abstract
Background: The respiratory illnesses caused by influenza virus can be dramatically reduced by vaccination. The current trivalent inactivated influenza vaccine is effective in eliciting systemic virus-specific antibodies sufficient to control viral replication. However, influenza protection generated after parenteral immunization could be improved by the induction of mucosal immune responses.
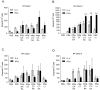
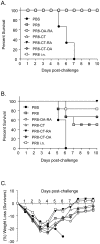
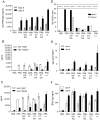
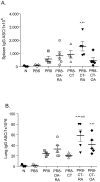
Methodology/principal findings: Transcutaneous immunization, a non-invasive vaccine delivery method, was used to investigate the quality, duration and effectiveness of the immune responses induced in the presence of inactivated influenza virus co-administered with retinoic acid or oleic acid. We observed an increased migration of dendritic cells to the draining lymph nodes after dermal vaccination. Here we demonstrate that this route of vaccine delivery in combination with certain immunomodulators can induce potent immune responses that result in long-term protective immunity. Additionally, mice vaccinated with inactivated virus in combination with retinoic acid show an enhanced sIgA antibody response, increased number of antibody secreting cells in the mucosal tissues, and protection from a higher influenza lethal dose.
Conclusions/significance: The present study demonstrates that transdermal administration of inactivated virus in combination with immunomodulators stimulates dendritic cell migration, results in long-lived systemic and mucosal responses that confer effective protective immunity.
Conflict of interest statement
Figures

Similar articles
-
An alphavirus-based adjuvant enhances serum and mucosal antibodies, T cells, and protective immunity to influenza virus in neonatal mice.J Virol. 2014 Aug;88(16):9182-96. doi: 10.1128/JVI.00327-14. Epub 2014 Jun 4. J Virol. 2014. PMID: 24899195 Free PMC article.
-
Bacterium-like particles supplemented with inactivated influenza antigen induce cross-protective influenza-specific antibody responses through intranasal administration.Vaccine. 2012 Jul 6;30(32):4884-91. doi: 10.1016/j.vaccine.2012.04.032. Epub 2012 Apr 23. Vaccine. 2012. PMID: 22537989
-
Intranasal administration of a flagellin-adjuvanted inactivated influenza vaccine enhances mucosal immune responses to protect mice against lethal infection.Vaccine. 2012 Jan 5;30(2):466-74. doi: 10.1016/j.vaccine.2011.10.058. Epub 2011 Oct 31. Vaccine. 2012. PMID: 22051136
-
Influenza virus: immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines.Scand J Immunol. 2004 Jan;59(1):1-15. doi: 10.1111/j.0300-9475.2004.01382.x. Scand J Immunol. 2004. PMID: 14723616 Review.
-
Cross-protective immunity against influenza virus infections induced by intranasal vaccination together with a TLR3-mucosal adjuvant.Hum Vaccin. 2011 Jan-Feb;7 Suppl:174-82. doi: 10.4161/hv.7.0.14584. Epub 2011 Jan 1. Hum Vaccin. 2011. PMID: 21321485 Review.
Cited by
-
The long-term immunogenicity of an inactivated split-virion 2009 pandemic influenza A H1N1 vaccine: Randomized, observer-masked, single-center clinical study.Results Immunol. 2012 Oct 9;2:184-9. doi: 10.1016/j.rinim.2012.10.001. eCollection 2012. Results Immunol. 2012. PMID: 24371582 Free PMC article.
-
What Lies Beneath: Antibody Dependent Natural Killer Cell Activation by Antibodies to Internal Influenza Virus Proteins.EBioMedicine. 2016 Jun;8:277-290. doi: 10.1016/j.ebiom.2016.04.029. Epub 2016 Apr 28. EBioMedicine. 2016. PMID: 27428437 Free PMC article.
-
Tailored vaccines targeting the elderly using whole inactivated influenza vaccines bearing cytokine immunomodulators.J Interferon Cytokine Res. 2014 Feb;34(2):129-39. doi: 10.1089/jir.2012.0119. Epub 2013 Oct 8. J Interferon Cytokine Res. 2014. PMID: 24102577 Free PMC article.
-
Nanoparticles for transcutaneous vaccination.Microb Biotechnol. 2012 Mar;5(2):156-67. doi: 10.1111/j.1751-7915.2011.00284.x. Epub 2011 Aug 19. Microb Biotechnol. 2012. PMID: 21854553 Free PMC article. Review.
-
Influenza Chimeric Protein (3M2e-3HA2-NP) Adjuvanted with PGA/Alum Confers Cross-Protection against Heterologous Influenza A Viruses.J Microbiol Biotechnol. 2021 Feb 28;31(2):304-316. doi: 10.4014/jmb.2011.11029. J Microbiol Biotechnol. 2021. PMID: 33263336 Free PMC article.
References
-
- Bush RM, Bender CA, Subbarao K, Cox NJ, Fitch WM. Predicting the evolution of human influenza A. Science. 1999;286:1921–1925. - PubMed
-
- Streilein JW. Skin-associated lymphoid tissues (SALT): origins and functions. J Invest Dermatol. 1983;80(Suppl):12s–16s. - PubMed
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

