Glial glucokinase expression in adult and post-natal development of the hypothalamic region
- PMID: 20531973
- PMCID: PMC2881537
- DOI: 10.1042/AN20090059
Glial glucokinase expression in adult and post-natal development of the hypothalamic region
Abstract
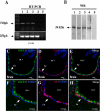
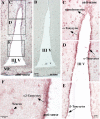
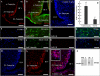
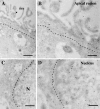
It has recently been proposed that hypothalamic glial cells sense glucose levels and release lactate as a signal to activate adjacent neurons. GK (glucokinase), the hexokinase involved in glucose sensing in pancreatic beta-cells, is also expressed in the hypothalamus. However, it has not been clearly determined if glial and/or neuronal cells express this protein. Interestingly, tanycytes, the glia that cover the ventricular walls of the hypothalamus, are in contact with CSF (cerebrospinal fluid), the capillaries of the arcuate nucleus and adjacent neurons; this would be expected for a system that can detect and communicate changes in glucose concentration. Here, we demonstrated by Western-blot analysis, QRT-PCR [quantitative RT-PCR (reverse transcription-PCR)] and in situ hybridization that GK is expressed in tanycytes. Confocal microscopy and immuno-ultrastructural analysis revealed that GK is localized in the nucleus and cytoplasm of beta1-tanycytes. Furthermore, GK expression increased in these cells during the second week of post-natal development. Based on this evidence, we propose that tanycytes mediate, at least in part, the mechanism by which the hypothalamus detects changes in glucose concentrations.
Keywords: CSF, cerebrospinal fluid; DIG, digoxigenin; DTT, dithiothreitol; GFAP, glial fibrillary acidic protein; GK, glucokinase; GKRP, GK regulatory protein; GLUT2, glucose transporter 2; RT–PCR, reverse transcription–PCR; TFIIB, transcription factor IIB; arcuate nucleus; brain; glia; glucokinase; glucosensing; tanycyte.
Figures

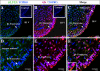
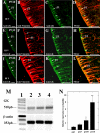
Similar articles
-
Dynamic localization of glucokinase and its regulatory protein in hypothalamic tanycytes.PLoS One. 2014 Apr 16;9(4):e94035. doi: 10.1371/journal.pone.0094035. eCollection 2014. PLoS One. 2014. PMID: 24739934 Free PMC article.
-
When a Little Bit More Makes the Difference: Expression Levels of GKRP Determines the Subcellular Localization of GK in Tanycytes.Front Neurosci. 2019 Mar 29;13:275. doi: 10.3389/fnins.2019.00275. eCollection 2019. Front Neurosci. 2019. PMID: 30983961 Free PMC article.
-
Localization of glucokinase gene expression in the rat brain.Diabetes. 2000 May;49(5):693-700. doi: 10.2337/diabetes.49.5.693. Diabetes. 2000. PMID: 10905475
-
The role of tanycytes in hypothalamic glucosensing.J Cell Mol Med. 2015 Jul;19(7):1471-82. doi: 10.1111/jcmm.12590. Epub 2015 Jun 17. J Cell Mol Med. 2015. PMID: 26081217 Free PMC article. Review.
-
Hypothalamic tanycytes: a key component of brain-endocrine interaction.Int Rev Cytol. 2005;247:89-164. doi: 10.1016/S0074-7696(05)47003-5. Int Rev Cytol. 2005. PMID: 16344112 Review.
Cited by
-
Inhibition of hypothalamic MCT1 expression increases food intake and alters orexigenic and anorexigenic neuropeptide expression.Sci Rep. 2016 Sep 28;6:33606. doi: 10.1038/srep33606. Sci Rep. 2016. PMID: 27677351 Free PMC article.
-
Adenovirus-mediated suppression of hypothalamic glucokinase affects feeding behavior.Sci Rep. 2017 Jun 16;7(1):3697. doi: 10.1038/s41598-017-03928-x. Sci Rep. 2017. PMID: 28623340 Free PMC article.
-
Glucokinase inhibitor glucosamine stimulates feeding and activates hypothalamic neuropeptide Y and orexin neurons.Behav Brain Res. 2011 Sep 12;222(1):274-8. doi: 10.1016/j.bbr.2011.03.043. Epub 2011 Apr 1. Behav Brain Res. 2011. PMID: 21440571 Free PMC article.
-
A sweet taste receptor-dependent mechanism of glucosensing in hypothalamic tanycytes.Glia. 2017 May;65(5):773-789. doi: 10.1002/glia.23125. Epub 2017 Feb 16. Glia. 2017. PMID: 28205335 Free PMC article.
-
A Short-Term Sucrose Diet Impacts Cell Proliferation of Neural Precursors in the Adult Hypothalamus.Nutrients. 2022 Jun 21;14(13):2564. doi: 10.3390/nu14132564. Nutrients. 2022. PMID: 35807744 Free PMC article.
References
-
- Akmayev IG, Popov AP. Morphological aspects of the hypothalamic–hypophyseal system. VII. The tanycytes: their relation to the hypophyseal adrenocorticotrophic function. An ultrastructural study. Cell Tissue Res. 1977;180:263–282. - PubMed
-
- Alvarez E, Roncero I, Chowen JA, Vazquez P, Blazquez E. Evidence that glucokinase regulatory protein is expressed and interacts with glucokinase in rat brain. J Neurochem. 2002;80:45–53. - PubMed
-
- Bady I, Marty N, Dallaporta M, Emery M, Gyger J, Tarussio D, Foretz M, Thorens B. Evidence from glut2-null mice that glucose is a critical physiological regulator of feeding. Diabetes. 2006;55:988–995. - PubMed
-
- Bali D, Svetlanov A, Lee HW, Fusco-DeMane D, Leiser M, Li B, Barzilai N, Surana M, Hou H, Fleischer N, DePinho R, Rossetti L, Efrat S. Animal model for maturity-onset diabetes of the young generated by disruption of the mouse glucokinase gene. J Biol Chem. 1995;270:21464–21467. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

