Prostaglandin E(2) is crucial in the response of podocytes to fluid flow shear stress
- PMID: 20531983
- PMCID: PMC2876242
- DOI: 10.1007/s12079-010-0088-9
Prostaglandin E(2) is crucial in the response of podocytes to fluid flow shear stress
Abstract
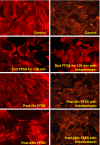
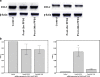
Podocytes play a key role in maintaining and modulating the filtration barrier of the glomerulus. Because of their location, podocytes are exposed to mechanical strain in the form of fluid flow shear stress (FFSS). Several human diseases are characterized by glomerular hyperfiltration, such as diabetes mellitus and hypertension. The response of podocytes to FFSS at physiological or pathological levels is not known. We exposed cultured podocytes to FFSS, and studied changes in actin cytoskeleton, prostaglandin E(2) (PGE(2)) production and expression of cyclooxygenase-1 and-2 (COX-1, COX-2). FFSS caused a reduction in transversal F-actin stress filaments and the appearance of cortical actin network in the early recovery period. Cells exhibited a pattern similar to control state by 24 h following FFSS without significant loss of podocytes or apoptosis. FFSS caused increased levels of PGE(2) as early as 30 min after onset of shear stress, levels that increased over time. PGE(2) production by podocytes at post-2 h and post-24 h was also significantly increased compared to control cells (p < 0.039 and 0.012, respectively). Intracellular PGE(2) synthesis and expression of COX-2 was increased at post-2 h following FFSS. The expression of COX-1 mRNA was unchanged. We conclude that podocytes are sensitive and responsive to FFSS, exhibiting morphological and physiological changes. We believe that PGE(2) plays an important role in mechanoperception in podocytes.
Keywords: Actin; Cyclooxygenase; Mechanical strain; Prostaglandin E2; Shear stress.
Figures






Similar articles
-
Cyclooxygenase-2, prostaglandin E2, and prostanoid receptor EP2 in fluid flow shear stress-mediated injury in the solitary kidney.Am J Physiol Renal Physiol. 2014 Dec 15;307(12):F1323-33. doi: 10.1152/ajprenal.00335.2014. Epub 2014 Sep 18. Am J Physiol Renal Physiol. 2014. PMID: 25234310
-
Mechanotransduction signaling in podocytes from fluid flow shear stress.Am J Physiol Renal Physiol. 2018 Jan 1;314(1):F22-F34. doi: 10.1152/ajprenal.00325.2017. Epub 2017 Sep 6. Am J Physiol Renal Physiol. 2018. PMID: 28877882 Free PMC article.
-
Fluid flow shear stress over podocytes is increased in the solitary kidney.Nephrol Dial Transplant. 2014 Jan;29(1):65-72. doi: 10.1093/ndt/gft387. Epub 2013 Oct 28. Nephrol Dial Transplant. 2014. PMID: 24166460 Free PMC article.
-
Hyperfiltration-associated biomechanical forces in glomerular injury and response: Potential role for eicosanoids.Prostaglandins Other Lipid Mediat. 2017 Sep;132:59-68. doi: 10.1016/j.prostaglandins.2017.01.003. Epub 2017 Jan 17. Prostaglandins Other Lipid Mediat. 2017. PMID: 28108282 Free PMC article. Review.
-
Role of biomechanical forces in hyperfiltration-mediated glomerular injury in congenital anomalies of the kidney and urinary tract.Nephrol Dial Transplant. 2017 May 1;32(5):759-765. doi: 10.1093/ndt/gfw430. Nephrol Dial Transplant. 2017. PMID: 28339567 Free PMC article. Review.
Cited by
-
Concerted EP2 and EP4 Receptor Signaling Stimulates Autocrine Prostaglandin E2 Activation in Human Podocytes.Cells. 2020 May 19;9(5):1256. doi: 10.3390/cells9051256. Cells. 2020. PMID: 32438662 Free PMC article.
-
Mechanical challenges and cytoskeletal impairments in focal segmental glomerulosclerosis.Am J Physiol Renal Physiol. 2018 May 1;314(5):F921-F925. doi: 10.1152/ajprenal.00641.2017. Epub 2018 Jan 24. Am J Physiol Renal Physiol. 2018. PMID: 29363327 Free PMC article. Review.
-
Upregulated proteoglycan-related signaling pathways in fluid flow shear stress-treated podocytes.Am J Physiol Renal Physiol. 2020 Aug 1;319(2):F312-F322. doi: 10.1152/ajprenal.00183.2020. Epub 2020 Jul 6. Am J Physiol Renal Physiol. 2020. PMID: 32628542 Free PMC article.
-
Urinary prostaglandin E2 is a biomarker of early adaptive hyperfiltration in solitary functioning kidney.Prostaglandins Other Lipid Mediat. 2020 Feb;146:106403. doi: 10.1016/j.prostaglandins.2019.106403. Epub 2019 Dec 12. Prostaglandins Other Lipid Mediat. 2020. PMID: 31838197 Free PMC article.
-
Hyperfiltration-mediated Injury in the Remaining Kidney of a Transplant Donor.Transplantation. 2018 Oct;102(10):1624-1635. doi: 10.1097/TP.0000000000002304. Transplantation. 2018. PMID: 29847501 Free PMC article. Review.
References
-
- Ajubi NE, Lkein-Nulend J, Alblas MJ, et al. Signal transduction pathways involved in fluid flow-induced PGE2 production by cultured osteocytes. Am J Physiology. 1997;276:E171–E178. - PubMed
-
- Arendshorst WJ, Navare LG. (1993) Renal circulation and glomerular hemodynamics. In: Schrier RW, Gottschalk CW (eds). Diseases of the kidney, 6th edn. Little Brown and Company, pp 59–106
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

