Hypoxia activates the K-ras proto-oncogene to stimulate angiogenesis and inhibit apoptosis in colon cancer cells
- PMID: 20532039
- PMCID: PMC2881039
- DOI: 10.1371/journal.pone.0010966
Hypoxia activates the K-ras proto-oncogene to stimulate angiogenesis and inhibit apoptosis in colon cancer cells
Abstract
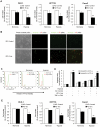
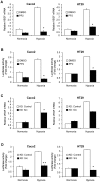
The KRAS proto-oncogene plays a key role in the development of many human tumors and is commonly activated by somatic mutation or signaling through specific growth factor receptors. However, the interaction between the micro-environment and K-ras activity has not been defined. Hypoxia invariably develops as tumors outgrow their supply of oxygen. A series of well-orchestrated cellular adaptations occur that stimulate angiogenesis and enhance survival of the tumor in hypoxic conditions. Our previous studies demonstrated that mutant KRAS alleles can interact with hypoxia to induce vascular endothelial growth factor (VEGF) in colon cancer. We sought to determine whether similar hypoxic responses are also present in tumors without a KRAS mutation. Hypoxia consistently increased the levels of activated, GTP-bound K-ras in colon cancer cell lines with a wild-type KRAS gene, and this depended upon the activation of c-Src. Inhibition of c-Src by PP2 treatment or siRNA knockdown blocked the hypoxic activation of K-ras. This activation of K-ras did not depend upon EGFR and resulted in the phosphorylation of Akt and induction of VEGF expression. In addition, activation of K-ras significantly blocked apoptosis in hypoxic conditions. These studies reveal a unique adaptive mechanism in hypoxia that activates K-ras signaling in the absence of a mutant KRAS oncogene.
Conflict of interest statement
Figures




References
-
- Vaupel P, Mayer A. Hypoxia in cancer: Significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26:225–239. - PubMed
-
- Zhang X, Gaspard JP, Chung DC. Regulation of vascular endothelial growth factor by the Wnt and K-Ras pathways in colonic neoplasia. Cancer Res. 2001;61:6050–6054. - PubMed
-
- Mizukami Y, Jo WS, Duerr EM, Gala M, Li J, et al. Induction of interleukin-8 preserves the angiogenic response in HIF-1alpha-deficient colon cancer cells. Nat Med. 2005;11:992–997. - PubMed
-
- Mizukami Y, Fujiki K, Duerr EM, Gala M, Jo WS, et al. Hypoxic regulation of vascular endothelial growth factor through the induction of phosphatidylinositol 3-kinase/Rho/ROCK and c-Myc. J Biol Chem. 2006;281:13957–13963. - PubMed
-
- Boguski MS, Mccormick F. Proteins regulating ras and its relatives. Nature. 1993;366:643–654. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

