Prenatal inflammation-induced hypoferremia alters dopamine function in the adult offspring in rat: relevance for schizophrenia
- PMID: 20532043
- PMCID: PMC2881043
- DOI: 10.1371/journal.pone.0010967
Prenatal inflammation-induced hypoferremia alters dopamine function in the adult offspring in rat: relevance for schizophrenia
Abstract
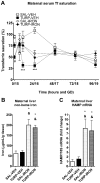
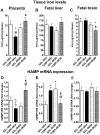
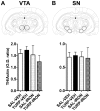
Maternal infection during pregnancy has been associated with increased incidence of schizophrenia in the adult offspring. Mechanistically, this has been partially attributed to neurodevelopmental disruption of the dopamine neurons, as a consequence of exacerbated maternal immunity. In the present study we sought to target hypoferremia, a cytokine-induced reduction of serum non-heme iron, which is common to all types of infections. Adequate iron supply to the fetus is fundamental for the development of the mesencephalic dopamine neurons and disruption of this following maternal infection can affect the offspring's dopamine function. Using a rat model of localized injury induced by turpentine, which triggers the innate immune response and inflammation, we investigated the effects of maternal iron supplementation on the offspring's dopamine function by assessing behavioral responses to acute and repeated administration of the dopamine indirect agonist, amphetamine. In addition we measured protein levels of tyrosine hydroxylase, and tissue levels of dopamine and its metabolites, in ventral tegmental area, susbtantia nigra, nucleus accumbens, dorsal striatum and medial prefrontal cortex. Offspring of turpentine-treated mothers exhibited greater responses to a single amphetamine injection and enhanced behavioral sensitization following repeated exposure to this drug, when compared to control offspring. These behavioral changes were accompanied by increased baseline levels of tyrosine hydroxylase, dopamine and its metabolites, selectively in the nucleus accumbens. Both, the behavioral and neurochemical changes were prevented by maternal iron supplementation. Localized prenatal inflammation induced a deregulation in iron homeostasis, which resulted in fundamental alterations in dopamine function and behavioral alterations in the adult offspring. These changes are characteristic of schizophrenia symptoms in humans.
Conflict of interest statement
Figures


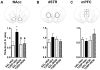

Similar articles
-
Leptin and interleukin-6 alter the function of mesolimbic dopamine neurons in a rodent model of prenatal inflammation.Psychoneuroendocrinology. 2012 Jul;37(7):956-69. doi: 10.1016/j.psyneuen.2011.11.003. Epub 2011 Nov 30. Psychoneuroendocrinology. 2012. PMID: 22133515
-
Maternal high fat diet during the perinatal period alters mesocorticolimbic dopamine in the adult rat offspring: reduction in the behavioral responses to repeated amphetamine administration.Psychopharmacology (Berl). 2008 Mar;197(1):83-94. doi: 10.1007/s00213-007-1008-4. Epub 2007 Nov 16. Psychopharmacology (Berl). 2008. PMID: 18004547
-
Early Development of Parvalbumin-, Somatostatin-, and Cholecystokinin-Expressing Neurons in Rat Brain following Prenatal Immune Activation and Maternal Iron Deficiency.Dev Neurosci. 2016;38(5):342-353. doi: 10.1159/000454677. Epub 2017 Feb 18. Dev Neurosci. 2016. PMID: 28214898
-
Maternal Immune Activation Disrupts Dopamine System in the Offspring.Int J Neuropsychopharmacol. 2016 Jul 5;19(7):pyw007. doi: 10.1093/ijnp/pyw007. Print 2016 Jul. Int J Neuropsychopharmacol. 2016. PMID: 26819283 Free PMC article.
-
Maternal Immune Activation and the Development of Dopaminergic Neurotransmission of the Offspring: Relevance for Schizophrenia and Other Psychoses.Front Psychiatry. 2020 Aug 21;11:852. doi: 10.3389/fpsyt.2020.00852. eCollection 2020. Front Psychiatry. 2020. PMID: 33061910 Free PMC article. Review.
Cited by
-
Dietary supplementation with n-3 fatty acids from weaning limits brain biochemistry and behavioural changes elicited by prenatal exposure to maternal inflammation in the mouse model.Transl Psychiatry. 2015 Sep 22;5(9):e641. doi: 10.1038/tp.2015.126. Transl Psychiatry. 2015. PMID: 26393487 Free PMC article.
-
Epigenetic and transgenerational mechanisms in infection-mediated neurodevelopmental disorders.Transl Psychiatry. 2017 May 2;7(5):e1113. doi: 10.1038/tp.2017.78. Transl Psychiatry. 2017. PMID: 28463237 Free PMC article. Review.
-
Vitamin D treatment during pregnancy prevents autism-related phenotypes in a mouse model of maternal immune activation.Mol Autism. 2017 Mar 7;8:9. doi: 10.1186/s13229-017-0125-0. eCollection 2017. Mol Autism. 2017. PMID: 28316773 Free PMC article.
-
Oxidative stress in schizophrenia: an integrated approach.Neurosci Biobehav Rev. 2011 Jan;35(3):878-93. doi: 10.1016/j.neubiorev.2010.10.008. Epub 2010 Oct 23. Neurosci Biobehav Rev. 2011. PMID: 20974172 Free PMC article. Review.
-
Schizophrenia: an integrated sociodevelopmental-cognitive model.Lancet. 2014 May 10;383(9929):1677-1687. doi: 10.1016/S0140-6736(13)62036-X. Epub 2013 Dec 6. Lancet. 2014. PMID: 24315522 Free PMC article. Review.
References
-
- Babulas V, Factor-Litvak P, Goetz R, Schaefer CA, Brown AS. Prenatal exposure to maternal genital and reproductive infections and adult schizophrenia. Am J Psychiatry. 2006;163:927–929. - PubMed
-
- Cui K, Ashdown H, Luheshi GN, Boksa P. Effects of prenatal immune activation on hippocampal neurogenesis in the rat. Schizophr Res. 2009;113:288–297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

