Expression of a Functional zipFv Antibody Fragment and Its Fusions with Alkaline Phosphatase in the Cytoplasm of an Escherichia coli
- PMID: 20532123
- PMCID: PMC2881423
- DOI: 10.4110/in.2010.10.2.35
Expression of a Functional zipFv Antibody Fragment and Its Fusions with Alkaline Phosphatase in the Cytoplasm of an Escherichia coli
Abstract
Background: Expression of recombinant antibodies and their derivatives fused with other functional molecules such as alkaline phosphatase in Escherichia coli is important in the development of molecular diagnostic reagents for biomedical research.
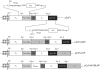
Methods: We investigated the possibility of applying a well-known Fos-Jun zipper to dimerize V(H) and V(L) fragments originated from the Fab clone (SP 112) that recognizes pyruvate dehydrogenase complex-E2 (PDC-E2), and demonstrated that the functional zipFv-112 and its alkaline phosphatase fusion molecules (zipFv-AP) can be produced in the cytoplasm of Origami(DE3) trxB gor mutant E. coli strain.
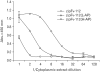
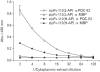
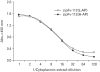
Results: The zipFv-AP fusion molecules exhibited higher antigen-binding signals than the zipFv up to a 10-fold under the same experimental conditions. However, conformation of the zipFv-AP seemed to be influenced by the location of an AP domain at the C-terminus of V(H) or V(L) domain [zipFv-112(H-AP) or zipFv-112(L-AP)], and inclusion of an AraC DNA binding domain at the C-terminus of V(H) of the zipFv-112(L-AP), termed zipFv-112(H-AD/L-AP), was also beneficial. Cytoplasmic co-expression of disulfide-binding isomerase C (DsbC) helped proper folding of the zipFv-112(H-AD/L-AP) but not significantly.
Conclusion: We believe that our zipFv constructs may serve as an excellent antibody format bi-functional antibody fragments that can be produced stably in the cytoplasm of E. coli.
Keywords: Alkaline phosphatase; DsbC; Fv; Leucine zipper; Recombinant antibody.
Conflict of interest statement
The author have no financial conflict of interest.
Figures


Similar articles
-
Thioredoxin fusions increase folding of single chain Fv antibodies in the cytoplasm of Escherichia coli: evidence that chaperone activity is the prime effect of thioredoxin.J Mol Biol. 2006 Mar 17;357(1):49-61. doi: 10.1016/j.jmb.2005.12.058. Epub 2006 Jan 6. J Mol Biol. 2006. PMID: 16427080
-
Engineering of a novel zipFv using leucine zipper motif against rabies virus glycoprotein G with improved protection potency in vivo.Immunol Lett. 2017 Jun;186:9-14. doi: 10.1016/j.imlet.2017.04.001. Epub 2017 Apr 4. Immunol Lett. 2017. PMID: 28389318
-
SKIK-zipbody-alkaline phosphatase, a novel antibody fusion protein expressed in Escherichia coli cytoplasm.J Biosci Bioeng. 2018 Dec;126(6):705-709. doi: 10.1016/j.jbiosc.2018.06.009. Epub 2018 Jul 25. J Biosci Bioeng. 2018. PMID: 30056072
-
Functional expression of single-chain variable fragment antibody against c-Met in the cytoplasm of Escherichia coli.Protein Expr Purif. 2006 May;47(1):203-9. doi: 10.1016/j.pep.2005.12.003. Epub 2005 Dec 28. Protein Expr Purif. 2006. PMID: 16414274
-
Solubility of disulfide-bonded proteins in the cytoplasm of Escherichia coli and its "oxidizing" mutant.World J Gastroenterol. 2005 Feb 21;11(7):1077-82. doi: 10.3748/wjg.v11.i7.1077. World J Gastroenterol. 2005. PMID: 15742420 Free PMC article.
References
-
- Morino K, Katsumi H, Akahori Y, Iba Y, Shinohara M, Ukai Y, Kohara Y, Kurosawa Y. Antibody fusions with fluorescent proteins: a versatile reagent for profiling protein expression. J Immunol Methods. 2001;257:175–184. - PubMed
-
- Pack P, Plückthun A. Miniantibodies use of amphipathic helices to produce functional, flexibly linked dimeric Fv fragments with high avidity in Escherichia coli. Biochemistry. 1992;31:1579–1584. - PubMed
-
- Horne C, Klein M, Polidoulis I, Dorrington KJ. Noncovalent association of heavy and light chains of human immunoglobulins. III. Specific interactions between VH and VL. J Immunol. 1982;129:660–664. - PubMed
-
- Jäger M, Plückthun A. Domain interactions in antibody Fv and scFv fragments: effects on unfolding kinetics and equilibria. FEBS Lett. 1999;462:307–312. - PubMed
-
- Jäger M, Plückthun A. Folding and assembly of an antibody Fv fragment, a heterodimer stabilized by antigen. J Mol Biol. 1999;285:2005–2019. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

