Roles of Host Nonhematopoietic Cells in Autoimmunity and Donor Cell Engraftment in Graft-versus-host Disease
- PMID: 20532124
- PMCID: PMC2881427
- DOI: 10.4110/in.2010.10.2.46
Roles of Host Nonhematopoietic Cells in Autoimmunity and Donor Cell Engraftment in Graft-versus-host Disease
Abstract
Background: Graft-versus-host disease (GVHD) is initiated when alloreactive donor T cells are primed by host APCs to undergo clonal expansion and maturation. Since there is a controversy regarding the role of nonhematopoietic cells in GVHD, we wanted to investigate the influence of MHC disparity on nonhematopoietic cells on the pathogenesis of GVHD in the MHC-haplomismatched C57BL/6 (H-2(b)) or DBA/2 (H-2(d))-->unirradiated (C57BL/6xDBA/2) F(1)(BDF(1); H-2(b/d)) murine model of acute GVHD (aGVHD) or chronic GVHD (cGVHD).
Methods: We generated (BDF(1)-->C57BL/6), (BDF(1)-->DBA/2), and (BDF(1)-->BDF(1)) chimeras and examined GVHD-related parameters and donor cell engraftment in those chimeras.
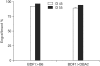
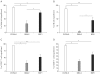
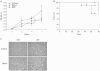
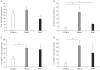
Results: Using this experimental system, we found that 1) severe aGVHD across MHC Ag barrier depends on the expression of nonhematopoietically rather than hematopoietically derived alloAgs for maximal GVHD manifestations; 2) host APCs were sufficient to break B cell tolerance to self molecules in cGVHD, whereas host APCs were insufficient to induce autoimmunity in aGVHD; 3) donor cell engraftment was greatly enhanced in the host with MHC-matched nonhematopoietic cells.
Conclusion: Taken together, our results provide an insight into how MHC disparity on GVHD target organs contribute to the pathogenesis of GVHD.
Keywords: Autoimmunity; Chimera; Graft-versus-host disease; Immune tolerance.
Conflict of interest statement
The author have no financial conflict of interest.
Figures

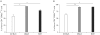
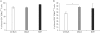
References
-
- Welniak LA, Blazar BR, Murphy WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu Rev Immunol. 2007;25:139–170. - PubMed
-
- Via CS, Sharrow SO, Shearer GM. Role of cytotoxic T lymphocytes in the prevention of lupus-like disease disease occurring in a murine model of graft-vs-host disease. J Immunol. 1987;139:1840–1849. - PubMed
-
- Rus V, Svetic A, Nguyen P, Gause WC, Via CS. Kinetics of Th1 and Th2 cytokine production during the early course of acute and chronic murine graft-versus-host disease. J Immunol. 1995;155:2396–2406. - PubMed
-
- Kim J, Choi WS, Kang H, Kim HJ, Suh J-H, Sakguchi S, Kwon B. Conversion of alloantigen-specific CD8+ T cell anergy to CD8+ T cell priming through in vivo ligation of glucocorticoid-induced TNF receptor. J Immunol. 2006;176:5223–5231. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

