Q344ter mutation causes mislocalization of rhodopsin molecules that are catalytically active: a mouse model of Q344ter-induced retinal degeneration
- PMID: 20532191
- PMCID: PMC2880002
- DOI: 10.1371/journal.pone.0010904
Q344ter mutation causes mislocalization of rhodopsin molecules that are catalytically active: a mouse model of Q344ter-induced retinal degeneration
Abstract
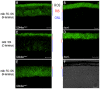
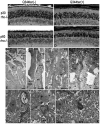
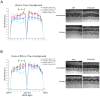
Q344ter is a naturally occurring rhodopsin mutation in humans that causes autosomal dominant retinal degeneration through mechanisms that are not fully understood, but are thought to involve an early termination that removed the trafficking signal, QVAPA, leading to its mislocalization in the rod photoreceptor cell. To better understand the disease mechanism(s), transgenic mice that express Q344ter were generated and crossed with rhodopsin knockout mice. Dark-reared Q344ter(rho+/-) mice exhibited retinal degeneration, demonstrating that rhodopsin mislocalization caused photoreceptor cell death. This degeneration is exacerbated by light-exposure and is correlated with the activation of transducin as well as other G-protein signaling pathways. We observed numerous sub-micrometer sized vesicles in the inter-photoreceptor space of Q344ter(rho+/-) and Q344ter(rho-/-) retinas, similar to that seen in another rhodopsin mutant, P347S. Whereas light microscopy failed to reveal outer segment structures in Q344ter(rho-/-) rods, shortened and disorganized rod outer segment structures were visible using electron microscopy. Thus, some Q344ter molecules trafficked to the outer segment and formed disc structures, albeit inefficiently, in the absence of full length wildtype rhodopsin. These findings helped to establish the in vivo role of the QVAPA domain as well as the pathways leading to Q344ter-induced retinal degeneration.
Conflict of interest statement
Figures




Similar articles
-
Retinal degeneration in humanized mice expressing mutant rhodopsin under the control of the endogenous murine promoter.Exp Eye Res. 2022 Feb;215:108893. doi: 10.1016/j.exer.2021.108893. Epub 2021 Dec 14. Exp Eye Res. 2022. PMID: 34919893
-
A rhodopsin gene mutation responsible for autosomal dominant retinitis pigmentosa results in a protein that is defective in localization to the photoreceptor outer segment.J Neurosci. 1994 Oct;14(10):5818-33. doi: 10.1523/JNEUROSCI.14-10-05818.1994. J Neurosci. 1994. PMID: 7523628 Free PMC article.
-
Phenotypic characterization of P23H and S334ter rhodopsin transgenic rat models of inherited retinal degeneration.Exp Eye Res. 2018 Feb;167:56-90. doi: 10.1016/j.exer.2017.10.023. Epub 2017 Nov 6. Exp Eye Res. 2018. PMID: 29122605 Free PMC article.
-
Transport of truncated rhodopsin and its effects on rod function and degeneration.Invest Ophthalmol Vis Sci. 2007 Jun;48(6):2868-76. doi: 10.1167/iovs.06-0035. Invest Ophthalmol Vis Sci. 2007. PMID: 17525223 Free PMC article.
-
Defective trafficking of rhodopsin and its role in retinal degenerations.Int Rev Cell Mol Biol. 2012;293:1-44. doi: 10.1016/B978-0-12-394304-0.00006-3. Int Rev Cell Mol Biol. 2012. PMID: 22251557 Review.
Cited by
-
Disrupted Plasma Membrane Protein Homeostasis in a Xenopus Laevis Model of Retinitis Pigmentosa.J Neurosci. 2019 Jul 10;39(28):5581-5593. doi: 10.1523/JNEUROSCI.3025-18.2019. Epub 2019 May 6. J Neurosci. 2019. PMID: 31061086 Free PMC article.
-
Proinflammatory Pathways Are Activated in the Human Q344X Rhodopsin Knock-In Mouse Model of Retinitis Pigmentosa.Biomolecules. 2021 Aug 6;11(8):1163. doi: 10.3390/biom11081163. Biomolecules. 2021. PMID: 34439829 Free PMC article.
-
The molecular and cellular basis of rhodopsin retinitis pigmentosa reveals potential strategies for therapy.Prog Retin Eye Res. 2018 Jan;62:1-23. doi: 10.1016/j.preteyeres.2017.10.002. Epub 2017 Oct 16. Prog Retin Eye Res. 2018. PMID: 29042326 Free PMC article. Review.
-
Sustained protection against photoreceptor degeneration in tubby mice by intravitreal injection of nanoceria.Biomaterials. 2012 Dec;33(34):8771-81. doi: 10.1016/j.biomaterials.2012.08.030. Epub 2012 Sep 6. Biomaterials. 2012. PMID: 22959465 Free PMC article.
-
Drug screening with zebrafish visual behavior identifies carvedilol as a potential treatment for an autosomal dominant form of retinitis pigmentosa.Sci Rep. 2021 Jun 1;11(1):11432. doi: 10.1038/s41598-021-89482-z. Sci Rep. 2021. PMID: 34075074 Free PMC article.
References
-
- Rivolta C, Sharon D, DeAngelis MM, Dryja TP. Retinitis pigmentosa and allied diseases: numerous diseases, genes, and inheritance patterns. Hum Mol Genet. 2002;11:1219–1227. - PubMed
-
- Wang DY, Chan WM, Tam PO, Baum L, Lam DS, et al. Gene mutations in retinitis pigmentosa and their clinical implications. Clin Chim Acta. 2005;351:5–16. - PubMed
-
- Gregory-Evans K, Bhattacharya SS. Genetic blindness: current concepts in the pathogenesis of human outer retinal dystrophies. Trends Genet. 1998;14:103–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

