The epigenetic landscape of latent Kaposi sarcoma-associated herpesvirus genomes
- PMID: 20532208
- PMCID: PMC2880564
- DOI: 10.1371/journal.ppat.1000935
The epigenetic landscape of latent Kaposi sarcoma-associated herpesvirus genomes
Abstract
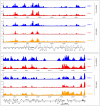
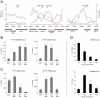
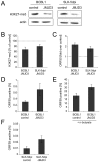
Herpesvirus latency is generally thought to be governed by epigenetic modifications, but the dynamics of viral chromatin at early timepoints of latent infection are poorly understood. Here, we report a comprehensive spatial and temporal analysis of DNA methylation and histone modifications during latent infection with Kaposi Sarcoma-associated herpesvirus (KSHV), the etiologic agent of Kaposi Sarcoma and primary effusion lymphoma (PEL). By use of high resolution tiling microarrays in conjunction with immunoprecipitation of methylated DNA (MeDIP) or modified histones (chromatin IP, ChIP), our study revealed highly distinct landscapes of epigenetic modifications associated with latent KSHV infection in several tumor-derived cell lines as well as de novo infected endothelial cells. We find that KSHV genomes are subject to profound methylation at CpG dinucleotides, leading to the establishment of characteristic global DNA methylation patterns. However, such patterns evolve slowly and thus are unlikely to control early latency. In contrast, we observed that latency-specific histone modification patterns were rapidly established upon a de novo infection. Our analysis furthermore demonstrates that such patterns are not characterized by the absence of activating histone modifications, as H3K9/K14-ac and H3K4-me3 marks were prominently detected at several loci, including the promoter of the lytic cycle transactivator Rta. While these regions were furthermore largely devoid of the constitutive heterochromatin marker H3K9-me3, we observed rapid and widespread deposition of H3K27-me3 across latent KSHV genomes, a bivalent modification which is able to repress transcription in spite of the simultaneous presence of activating marks. Our findings suggest that the modification patterns identified here induce a poised state of repression during viral latency, which can be rapidly reversed once the lytic cycle is induced.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Pellett PE, Roizman B. The family herpesviridae: A brief introduction. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, editors. Fields virology. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 2381–2398.
-
- Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186–1191. - PubMed
-
- Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266:1865–1869. - PubMed
-
- Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, et al. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood. 1995;86:1276–1280. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

