A feed-forward circuit linking wingless, fat-dachsous signaling, and the warts-hippo pathway to Drosophila wing growth
- PMID: 20532238
- PMCID: PMC2879410
- DOI: 10.1371/journal.pbio.1000386
A feed-forward circuit linking wingless, fat-dachsous signaling, and the warts-hippo pathway to Drosophila wing growth
Abstract
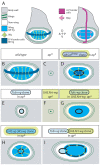
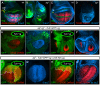
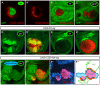
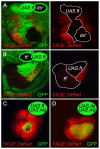
During development, the Drosophila wing primordium undergoes a dramatic increase in cell number and mass under the control of the long-range morphogens Wingless (Wg, a Wnt) and Decapentaplegic (Dpp, a BMP). This process depends in part on the capacity of wing cells to recruit neighboring, non-wing cells into the wing primordium. Wing cells are defined by activity of the selector gene vestigial (vg) and recruitment entails the production of a vg-dependent "feed-forward signal" that acts together with morphogen to induce vg expression in neighboring non-wing cells. Here, we identify the protocadherins Fat (Ft) and Dachsous (Ds), the Warts-Hippo tumor suppressor pathway, and the transcriptional co-activator Yorkie (Yki, a YES associated protein, or YAP) as components of the feed-forward signaling mechanism, and we show how this mechanism promotes wing growth in response to Wg. We find that vg generates the feed-forward signal by creating a steep differential in Ft-Ds signaling between wing and non-wing cells. This differential down-regulates Warts-Hippo pathway activity in non-wing cells, leading to a burst of Yki activity and the induction of vg in response to Wg. We posit that Wg propels wing growth at least in part by fueling a wave front of Ft-Ds signaling that propagates vg expression from one cell to the next.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

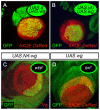
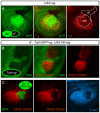

Comment in
-
Vestigial rides a fat/dachsous wave in wing development.PLoS Biol. 2010 Jun 1;8(6):e1000389. doi: 10.1371/journal.pbio.1000389. PLoS Biol. 2010. PMID: 20532242 Free PMC article. No abstract available.
Similar articles
-
A unified mechanism for the control of Drosophila wing growth by the morphogens Decapentaplegic and Wingless.PLoS Biol. 2021 Mar 3;19(3):e3001111. doi: 10.1371/journal.pbio.3001111. eCollection 2021 Mar. PLoS Biol. 2021. PMID: 33657096 Free PMC article.
-
Fat and Wingless signaling oppositely regulate epithelial cell-cell adhesion and distal wing development in Drosophila.Development. 2006 Mar;133(5):925-35. doi: 10.1242/dev.02243. Epub 2006 Feb 1. Development. 2006. PMID: 16452097
-
Fat/Dachsous Signaling Promotes Drosophila Wing Growth by Regulating the Conformational State of the NDR Kinase Warts.Dev Cell. 2015 Dec 21;35(6):737-49. doi: 10.1016/j.devcel.2015.11.027. Dev Cell. 2015. PMID: 26702832 Free PMC article.
-
Formation and maintenance of morphogen gradients: an essential role for the endomembrane system in Drosophila melanogaster wing development.Fly (Austin). 2011 Jul-Sep;5(3):266-71. doi: 10.4161/fly.5.3.16542. Epub 2011 Jul 1. Fly (Austin). 2011. PMID: 21654212 Review.
-
Upstream paths for Hippo signaling in Drosophila organ development.BMB Rep. 2018 Mar;51(3):134-142. doi: 10.5483/bmbrep.2018.51.3.027. BMB Rep. 2018. PMID: 29397870 Free PMC article. Review.
Cited by
-
odd-skipped genes and lines organize the notum anterior-posterior axis using autonomous and non-autonomous mechanisms.Mech Dev. 2012 Jul;129(5-8):147-61. doi: 10.1016/j.mod.2012.05.001. Epub 2012 May 14. Mech Dev. 2012. PMID: 22613630 Free PMC article.
-
Salvador-Warts-Hippo pathway in a developmental checkpoint monitoring helix-loop-helix proteins.Dev Cell. 2015 Jan 26;32(2):191-202. doi: 10.1016/j.devcel.2014.12.002. Epub 2015 Jan 8. Dev Cell. 2015. PMID: 25579975 Free PMC article.
-
The Hippo pathway effector Yki downregulates Wg signaling to promote retinal differentiation in the Drosophila eye.Development. 2015 Jun 1;142(11):2002-13. doi: 10.1242/dev.117358. Epub 2015 May 14. Development. 2015. PMID: 25977365 Free PMC article.
-
PANET: a GPU-based tool for fast parallel analysis of robustness dynamics and feed-forward/feedback loop structures in large-scale biological networks.PLoS One. 2014 Jul 24;9(7):e103010. doi: 10.1371/journal.pone.0103010. eCollection 2014. PLoS One. 2014. PMID: 25058310 Free PMC article.
-
Expanding signaling-molecule wavefront model of cell polarization in the Drosophila wing primordium.PLoS Comput Biol. 2017 Jul 3;13(7):e1005610. doi: 10.1371/journal.pcbi.1005610. eCollection 2017 Jul. PLoS Comput Biol. 2017. PMID: 28671940 Free PMC article.
References
-
- Zecca M, Basler K, Struhl G. Sequential organizing activities of engrailed, hedgehog and decapentaplegic in the Drosophila wing. Development. 1995;121:2265–2278. - PubMed
-
- Nellen D, Burke R, Struhl G, Basler K. Direct and long-range action of a DPP morphogen gradient. Cell. 1996;85:357–368. - PubMed
-
- Lecuit T, Brook W. J, Ng M, Calleja M, Sun H, et al. Two distinct mechanisms for long-range patterning by Decapentaplegic in the Drosophila wing. Nature. 1996;381:387–393. - PubMed
-
- Zecca M, Basler K, Struhl G. Direct and long-range action of a wingless morphogen gradient. Cell. 1996;87:833–844. - PubMed
-
- Neumann C. J, Cohen S. M. Long-range action of Wingless organizes the dorsal-ventral axis of the Drosophila wing. Development. 1997;124:871–880. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

