Kinetics of neutralizing antibodies in patients naturally infected by H5N1 virus
- PMID: 20532246
- PMCID: PMC2879427
- DOI: 10.1371/journal.pone.0010864
Kinetics of neutralizing antibodies in patients naturally infected by H5N1 virus
Abstract
Background: Little is known about the kinetics of anti-H5 neutralizing antibodies in naturally H5N1-infected patients with severe clinical illness or asymptomatic infection.
Methods: Using H5N1 microneutralisation (MN) and H5-pseudotype particle-based microneutralisation assays (H5pp) we analyzed sera sequentially obtained from 11 severely ill patients diagnosed by RT-PCR (follow-up range 1-139 weeks of disease onset) and 31 asymptomatically infected individuals detected in a sero-epidemiological study after exposure to H5N1 virus (follow-up range: 1-2 month-11 months after exposure).
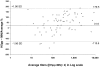
Results: Of 44 sera from 11 patients with H5N1 disease, 70% tested positive by MN (antibody titre > or = 80) after 2 weeks and 100% were positive by 3 weeks after disease onset. The geometric mean MN titers in severely ill patients were 540 at 1-2 months and 173 at 10-12 months and thus were higher than the titers from asymptomatic individuals (149 at 1-2 months, 62.2 at 10-12 months). Fractional polynomial regression analysis demonstrated that in all severely ill patients, positive titers persisted beyond 2 years of disease onset, while 10 of 23 sera collected 10-11 months after exposure in asymptomatically infected individuals tested negative.
Conclusions: Our results indicate that people with asymptomatic H5N1 infection have lower H5N1 antibody titres compared to those with severe illness and that in many asymptomatically infected patients the antibody titer decreased to levels below the threshold of positivity within one year. These data are essential for the design and interpretation of sero-epidemiological studies.
Conflict of interest statement
Figures
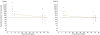
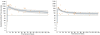
Similar articles
-
Heterosubtype neutralizing responses to influenza A (H5N1) viruses are mediated by antibodies to virus haemagglutinin.PLoS One. 2009 Nov 20;4(11):e7918. doi: 10.1371/journal.pone.0007918. PLoS One. 2009. PMID: 19936250 Free PMC article.
-
Safety and immunogenicity of a vero cell culture-derived whole-virus H5N1 influenza vaccine in chronically ill and immunocompromised patients.Clin Vaccine Immunol. 2014 Jun;21(6):867-76. doi: 10.1128/CVI.00065-14. Epub 2014 Apr 16. Clin Vaccine Immunol. 2014. PMID: 24739978 Free PMC article. Clinical Trial.
-
A sensitive retroviral pseudotype assay for influenza H5N1-neutralizing antibodies.Influenza Other Respir Viruses. 2007 May;1(3):105-12. doi: 10.1111/j.1750-2659.2007.00016.x. Influenza Other Respir Viruses. 2007. PMID: 19453415 Free PMC article.
-
Highly pathogenic avian influenza A(H5N1) mutants transmissible by air are susceptible to human and animal neutralizing antibodies.J Infect Dis. 2013 Oct 15;208(8):1315-9. doi: 10.1093/infdis/jit323. Epub 2013 Jul 18. J Infect Dis. 2013. PMID: 23868877 Free PMC article.
-
A human antibody recognizing a conserved epitope of H5 hemagglutinin broadly neutralizes highly pathogenic avian influenza H5N1 viruses.J Virol. 2012 Mar;86(6):2978-89. doi: 10.1128/JVI.06665-11. Epub 2012 Jan 11. J Virol. 2012. PMID: 22238297 Free PMC article.
Cited by
-
Seroprevalence of antibodies to avian influenza A (H5) and A (H9) viruses among market poultry workers, Hanoi, Vietnam, 2001.PLoS One. 2012;7(8):e43948. doi: 10.1371/journal.pone.0043948. Epub 2012 Aug 21. PLoS One. 2012. PMID: 22928049 Free PMC article.
-
Specificity, kinetics and longevity of antibody responses to avian influenza A(H7N9) virus infection in humans.J Infect. 2020 Mar;80(3):310-319. doi: 10.1016/j.jinf.2019.11.024. Epub 2020 Jan 16. J Infect. 2020. PMID: 31954742 Free PMC article.
-
More Than Just Gene Therapy Vectors: Lentiviral Vector Pseudotypes for Serological Investigation.Viruses. 2021 Jan 31;13(2):217. doi: 10.3390/v13020217. Viruses. 2021. PMID: 33572589 Free PMC article. Review.
-
Seroprevalence of H7N9 infection among humans: A systematic review and meta-analysis.Influenza Other Respir Viruses. 2020 Sep;14(5):587-595. doi: 10.1111/irv.12736. Epub 2020 Mar 10. Influenza Other Respir Viruses. 2020. PMID: 32157809 Free PMC article.
-
Suboptimal Humoral Immune Response against Influenza A(H7N9) Virus Is Related to Its Internal Genes.Clin Vaccine Immunol. 2015 Dec;22(12):1235-43. doi: 10.1128/CVI.00443-15. Epub 2015 Oct 7. Clin Vaccine Immunol. 2015. PMID: 26446420 Free PMC article.
References
-
- World Health Organization. (WHO). Cumulative number of confirmed human cases of avian influenza A/(H5N1) reported to WHO, 12 February. 2010. Available: http://www.who.int/csr/disease/avian_influenza/country/cases_table_2010_.... Accessed 2010 Feb 13.
-
- Vincent AL, Ma W, Lager KM, Janke BH, Richt JA. Swine influenza viruses a North American perspective. Adv Virus Res. 2009;72:127–154. - PubMed
-
- Bridges CB, Lim W, Hu-Primmer J, Sims L, Fukuda K, et al. Risk of influenza A (H5N1) Infection among poultry workers, Hong Kong, 1991-1998. J Infect Dis. 2002;185:1005–1010. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

