AMP kinase activation and glut4 translocation in isolated cardiomyocytes
- PMID: 20532430
- PMCID: PMC3734761
AMP kinase activation and glut4 translocation in isolated cardiomyocytes
Abstract
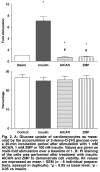
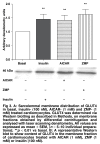
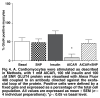
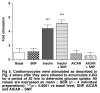
Activation of AMP-activated protein kinase (AMPK) results in glucose transporter 4 (GLUT4) translocation from the cytosol to the cell membrane, and glucose uptake in the skeletal muscles. This increased activation of AMPK can be stimulated by a pharmacological agent, AICAR (5' -aminoimidazole-4-carboxamide ribonucleoside), which is converted intracellularly into ZMP (5' -aminoimidazole-4-carboxamideribonucleosidephosphate), an AMP analogue. We utilised AICAR and ZMP to study GLUT4 translocation and glucose uptake in isolated cardiomyocytes. Adult ventricular cardiomyocytes were treated with AICAR or ZMP, and glucose uptake was measured via [3H] -2-deoxyglucose accumulation. PKB/Akt, AMPK and acetyl-CoA-carboxylase phosphorylation and GLUT4 translocation were detected by Western blotting or flow cytometry. AICAR and ZMP promoted AMPK phosphorylation. Neither drug increased glucose uptake but on the contrary, inhibited basal glucose uptake, although GLUT4 translocation from the cytosol to the membrane occurred. Using flow cytometry to detect the exofacial loop of the GLUT4 protein, we showed ineffective insertion in the membrane under these conditions. Supplementing with nitric oxide improved insertion in the membrane but not glucose uptake. We concluded that activation of AMPK via AICAR or ZMP was not sufficient to induce GLUT4-mediated glucose uptake in isolated cardiomyocytes. Nitric oxide plays a role in proper insertion of the protein in the membrane but not in glucose uptake.
Figures

Similar articles
-
5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside reduces glucose uptake via the inhibition of Na+/H+ exchanger 1 in isolated rat ventricular cardiomyocytes.Endocrinology. 2008 Apr;149(4):1490-8. doi: 10.1210/en.2007-1326. Epub 2008 Jan 10. Endocrinology. 2008. PMID: 18187546
-
Nitric oxide increases GLUT4 expression and regulates AMPK signaling in skeletal muscle.Am J Physiol Endocrinol Metab. 2007 Oct;293(4):E1062-8. doi: 10.1152/ajpendo.00045.2007. Epub 2007 Jul 31. Am J Physiol Endocrinol Metab. 2007. PMID: 17666490
-
Role of the nitric oxide pathway in AMPK-mediated glucose uptake and GLUT4 translocation in heart muscle.Am J Physiol Endocrinol Metab. 2004 Nov;287(5):E834-41. doi: 10.1152/ajpendo.00234.2004. Epub 2004 Jul 20. Am J Physiol Endocrinol Metab. 2004. PMID: 15265762
-
Calcium signaling recruits substrate transporters GLUT4 and CD36 to the sarcolemma without increasing cardiac substrate uptake.Am J Physiol Endocrinol Metab. 2014 Jul 15;307(2):E225-36. doi: 10.1152/ajpendo.00655.2013. Epub 2014 Jun 3. Am J Physiol Endocrinol Metab. 2014. PMID: 24895286
-
Prior treatment with the AMPK activator AICAR induces subsequently enhanced glucose uptake in isolated skeletal muscles from 24-month-old rats.Appl Physiol Nutr Metab. 2018 Aug;43(8):795-805. doi: 10.1139/apnm-2017-0858. Epub 2018 Mar 8. Appl Physiol Nutr Metab. 2018. PMID: 29518344 Free PMC article.
Cited by
-
The impact of an acute high polyphenol, high fiber meal with and without aerobic exercise on metabolism in middle-aged and older adults: A pilot study.Physiol Rep. 2025 Jun;13(11):e70312. doi: 10.14814/phy2.70312. Physiol Rep. 2025. PMID: 40443047 Free PMC article. Clinical Trial.
-
AICAR Protects against High Palmitate/High Insulin-Induced Intramyocellular Lipid Accumulation and Insulin Resistance in HL-1 Cardiac Cells by Inducing PPAR-Target Gene Expression.PPAR Res. 2015;2015:785783. doi: 10.1155/2015/785783. Epub 2015 Nov 16. PPAR Res. 2015. PMID: 26649034 Free PMC article.
-
The WWOX/HIF1A Axis Downregulation Alters Glucose Metabolism and Predispose to Metabolic Disorders.Int J Mol Sci. 2022 Mar 19;23(6):3326. doi: 10.3390/ijms23063326. Int J Mol Sci. 2022. PMID: 35328751 Free PMC article.
-
Flow cytometry protocol for GLUT4-myc detection on cell surfaces.Biosci Rep. 2024 Apr 24;44(4):BSR20231987. doi: 10.1042/BSR20231987. Biosci Rep. 2024. PMID: 38533799 Free PMC article.
-
Health position paper and redox perspectives on reactive oxygen species as signals and targets of cardioprotection.Redox Biol. 2023 Nov;67:102894. doi: 10.1016/j.redox.2023.102894. Epub 2023 Oct 6. Redox Biol. 2023. PMID: 37839355 Free PMC article. Review.
References
-
- Hardie DG, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: Metabolic sensors of the eukaryotic cell? A Rev Biochem. 1998;67:821–855. - PubMed
-
- Hardie DG, Carling D. The AMP-activated protein kinase – fuel gauge of the mammalian cell? Eur J Biochem. 1997;246(2):259–273. - PubMed
-
- Kemp BE, Mitchelhill KI, Stapleton D, Michell BJ, Chen Z-P, Witters LA. Dealing with energy demand: the AMP-activated protein kinase. TIBS. 1999;24:22–25. - PubMed
-
- Hardie DG, Hawley SA. AMP-activated protein kinase: the energy charge hypothesis revisited. Bioessays. 2001;23(12):1112–1119. - PubMed
-
- Chabowski A, Momken I, Coort SL, Calles-Escandon J, Tandon NN, Glatz JF. et al. Prolonged AMPK activation increases the expression of fatty acid transporters in cardiac myocytes and perfused hearts. Mol Cell Biochem. 2006;288(1-2):201–212. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

