Reproductive abnormalities in mice expressing omega-3 fatty acid desaturase in their mammary glands
- PMID: 20532624
- PMCID: PMC3051059
- DOI: 10.1007/s11248-010-9407-4
Reproductive abnormalities in mice expressing omega-3 fatty acid desaturase in their mammary glands
Abstract
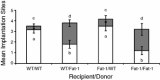
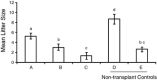
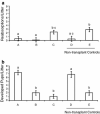
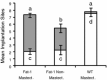
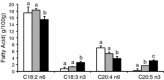
The Caenorhabditis elegans n-3 fatty acid desaturase (Fat-1) acts on a range of 18- and 20-carbon n-6 fatty acid substrates. Transgenic female mice expressing the Fat-1 gene under transcriptional control of the goat β-casein promoter produce milk phospholipids having elevated levels of n-3 polyunsaturated fatty acids (PUFA). However, females from this line were also observed to have impaired reproductive performance characterized by a smaller litter size (2.7 ± 0.6 vs. 7.2 ± 0.7; P < 0.05) than wildtype controls. While there is a close association between PUFA metabolism, prostaglandin biosynthesis, and fertility; reproductive problems in these mice were unanticipated given that the Fat-1 transgene is primarily expressed in the lactating mammary gland. Using multiple approaches it was found that Fat-1 mice have normal ovulation and fertilization rates; however fewer embryos were present in the uterus prior to implantation. Small litter size was also found to be partly attributable to a high incidence of post-implantation fetal resorptions. Embryo transfer experiments revealed that embryos developing from oocytes derived from transgenic ovaries had an increased rate of post-implantation resorption, regardless of the uterine genotype. Ovary transplantation between Fat-1 and C57BL/6 wildtype females revealed that non-ovarian factors also contributed to the smaller litter size phenotype. Finally, surgical removal of the mammary glands from juvenile Fat-1 mice increased the subsequent number of implantation sites per female, but did not lessen the high rate of post-implantation resorptions. In conclusion, we herein report on a system where an exogenous transgene expressed predominately in the mammary gland detrimentally affects female reproduction, suggesting that in certain circumstances the mammary gland may function as an endocrine regulator of reproductive performance.
Figures

Similar articles
-
Endogenous production and elevated levels of long-chain n-3 fatty acids in the milk of transgenic mice.J Dairy Sci. 2006 Aug;89(8):3195-201. doi: 10.3168/jds.S0022-0302(06)72594-2. J Dairy Sci. 2006. PMID: 16840637
-
Neonatal growth rate and development of mice raised on milk transgenically enriched with omega-3 fatty acids.Pediatr Res. 2007 Oct;62(4):412-6. doi: 10.1203/PDR.0b013e31813cbeea. Pediatr Res. 2007. PMID: 17667849
-
N-3 polyunsaturated fatty acids endogenously synthesized in fat-1 mice are enriched in the mammary gland.Lipids. 2006 Jan;41(1):35-9. doi: 10.1007/s11745-006-5067-9. Lipids. 2006. PMID: 16555469
-
Fatty Acid Desaturases, Polyunsaturated Fatty Acid Regulation, and Biotechnological Advances.Nutrients. 2016 Jan 4;8(1):23. doi: 10.3390/nu8010023. Nutrients. 2016. PMID: 26742061 Free PMC article. Review.
-
Genomic and functional characterization of polyunsaturated fatty acid biosynthesis in Caenorhabditis elegans.Lipids. 2001 Aug;36(8):761-6. doi: 10.1007/s11745-001-0782-9. Lipids. 2001. PMID: 11592725 Review.
Cited by
-
Elevated serum α-linolenic acid levels are associated with decreased chance of pregnancy after in vitro fertilization.Fertil Steril. 2011 Oct;96(4):880-3. doi: 10.1016/j.fertnstert.2011.07.1115. Epub 2011 Aug 15. Fertil Steril. 2011. PMID: 21840520 Free PMC article.
-
Polyunsaturated fatty acid derived signaling in reproduction and development: insights from Caenorhabditis elegans and Drosophila melanogaster.Mol Reprod Dev. 2013 Apr;80(4):244-59. doi: 10.1002/mrd.22167. Epub 2013 Mar 14. Mol Reprod Dev. 2013. PMID: 23440886 Free PMC article. Review.
-
Diverse and active roles for adipocytes during mammary gland growth and function.J Mammary Gland Biol Neoplasia. 2010 Sep;15(3):279-90. doi: 10.1007/s10911-010-9187-8. Epub 2010 Aug 19. J Mammary Gland Biol Neoplasia. 2010. PMID: 20717712 Free PMC article. Review.
-
Stress response pathways protect germ cells from omega-6 polyunsaturated fatty acid-mediated toxicity in Caenorhabditis elegans.Dev Biol. 2013 Jan 1;373(1):14-25. doi: 10.1016/j.ydbio.2012.10.002. Epub 2012 Oct 9. Dev Biol. 2013. PMID: 23064027 Free PMC article.
-
Fat-1 Transgene Is Associated With Improved Reproductive Outcomes.Endocrinology. 2018 Dec 1;159(12):3981-3992. doi: 10.1210/en.2018-00723. Endocrinology. 2018. PMID: 30403782 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

