Nuclear-cytoplasmic localization of acetyl coenzyme a synthetase-1 in the rat brain
- PMID: 20533355
- PMCID: PMC3047483
- DOI: 10.1002/cne.22373
Nuclear-cytoplasmic localization of acetyl coenzyme a synthetase-1 in the rat brain
Abstract
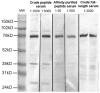
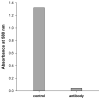
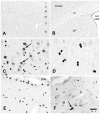
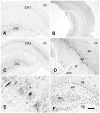
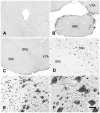
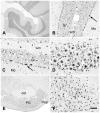
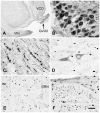
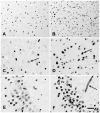
Acetyl coenzyme A synthetase-1 (AceCS1) catalyzes the synthesis of acetyl coenzyme A from acetate and coenzyme A and is thought to play diverse roles ranging from fatty acid synthesis to gene regulation. By using an affinity-purified antibody generated against an 18-mer peptide sequence of AceCS1 and a polyclonal antibody directed against recombinant AceCS1 protein, we examined the expression of AceCS1 in the rat brain. AceCS1 immunoreactivity in the adult rat brain was present predominantly in cell nuclei, with only light to moderate cytoplasmic staining in some neurons, axons, and oligodendrocytes. Some nonneuronal cell nuclei were very strongly immunoreactive, including those of some oligodendrocytes, whereas neuronal nuclei ranged from unstained to moderately stained. Both antibodies stained some neuronal cell bodies and axons, especially in the hindbrain. AceCS1 immunoreactivity was stronger and more widespread in the brains of 18-day-old rats than in adults, with increased expression in oligodendrocytes and neurons, including cortical pyramidal cells. Expression of AceCS1 was substantially up-regulated in neurons throughout the brain after controlled cortical impact injury. The strong AceCS1 expression observed in the nuclei of CNS cells during brain development and after injury is consistent with a role in nuclear histone acetylation and therefore the regulation of chromatin structure and gene expression. The cytoplasmic staining observed in some oligodendrocytes, especially during postnatal brain development, suggests an additional role in CNS lipid synthesis and myelination. Neuronal and axonal localization implicates AceCS1 in cytoplasmic acetylation reactions in some neurons.
(c) 2010 Wiley-Liss, Inc.
Figures









References
-
- Ariyannur PS, Madhavarao CN, Namboodiri AM. N-acetylaspartate synthesis in the brain: Mitochondria vs. microsomes. Brain Res. 2008;1227:34–41. - PubMed
-
- Arun P, Moffett JR, Namboodiri AM. Evidence for mitochondrial and cytoplasmic N-acetylaspartate synthesis in SH-SY5Y neuroblastoma cells. Neurochem Int. 2009;55(4):219–25. - PubMed
-
- Baurle J, Bruning G, Schemann M, Nishiike S, Guldin WO. Co-localization of glutamate, choline acetyltransferase and glycine in the mammalian vestibular ganglion and periphery. Neuroreport. 1999;10(17):3517–21. - PubMed
-
- Benjamin AM, Quastel JH. Acetylcholine synthesis in synaptosomes: mode of transfer of mitochondrial acetyl coenzyme A. Science. 1981;213(4515):1495–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

