Age changes in myelinated nerve fibers of the cingulate bundle and corpus callosum in the rhesus monkey
- PMID: 20533359
- PMCID: PMC2889619
- DOI: 10.1002/cne.22379
Age changes in myelinated nerve fibers of the cingulate bundle and corpus callosum in the rhesus monkey
Abstract
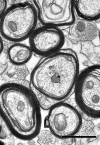
Aging is accompanied by deficits in cognitive function, which may be related to the vulnerability of myelinated nerve fibers to the normal process of aging. Loss of nerve fibers, together with age-related alterations in myelin sheath structure, may result in the inefficient and poorly coordinated conduction of neuronal signals. Until now, the ultrastructural analysis of cerebral white matter fiber tracts associated with frontal lobe areas critical in cognitive processing has been limited. In this study we analyzed the morphology and area number density of myelinated nerve fibers in the cingulate bundle and genu of the corpus callosum in behaviorally assessed young, middle aged, and old rhesus monkeys (Macaca mulatta). In both structures, normal aging results in a 20% decrease in the number of myelinated nerve fibers per unit area, while remaining nerve fibers exhibit an increasing frequency of degenerative changes in their myelin sheaths throughout middle and old age. Concomitantly, myelination continues in older monkeys, suggesting ongoing, albeit inadequate, reparative processes. Despite similar patterns of degeneration in both fiber tracts, only the age-related changes in the cingulate bundle correlate with declining cognitive function, underscoring its role as a critical corticocortical pathway linking the medial prefrontal, cingulate, and parahippocampal cortices in processes of working memory, recognition memory, and other higher cognitive faculties. These results further demonstrate the important role myelinated nerve fiber degeneration plays in the pathogenesis of age-related cognitive decline.
(c) 2010 Wiley-Liss, Inc.
Figures










References
-
- Albert MS. Cognitive Function. In: Albert MS, Moss MB, editors. Geriatric Neuropsychology. The Guilford Press; New York: 1988. pp. 33–53.
-
- Albert MS. Neuropsychological and neurophysiological changes in healthy adult humans across the age range. Neurobiol Aging. 1993;14:623–625. - PubMed
-
- Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: the major lobes and a parcellation of the temporal region. Neurobiol Aging. 2005;26:1245–1260. discussion 1279-1282. - PubMed
-
- Barbas H, Pandya DN. Topography of commissural fibers of the prefrontal cortex in the rhesus monkey. Exp Brain Res. 1984;55:187–191. - PubMed
-
- Blakemore WF. Observations on oligodendrocyte degeneration, the resolution of status spongiosus and remyelination in cuprizone intoxication in mice. J Neurocytol. 1972;1:413–426. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

