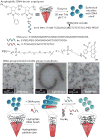
Programmable shape-shifting micelles
- PMID: 20533475
- PMCID: PMC4671773
- DOI: 10.1002/anie.201000265
Programmable shape-shifting micelles
Figures


Similar articles
-
Controlled nucleation of DNA metallization.Angew Chem Int Ed Engl. 2009;48(1):219-23. doi: 10.1002/anie.200803123. Angew Chem Int Ed Engl. 2009. PMID: 19040259 No abstract available.
-
Enhanced gene expression promoted by the quantized folding of pDNA within polyplex micelles.Biomaterials. 2012 Jan;33(1):325-32. doi: 10.1016/j.biomaterials.2011.09.046. Epub 2011 Oct 10. Biomaterials. 2012. PMID: 21993237
-
Self-assembled, thermosensitive micelles of a star block copolymer based on PMMA and PNIPAAm for controlled drug delivery.Biomaterials. 2007 Jan;28(1):99-107. doi: 10.1016/j.biomaterials.2006.08.030. Epub 2006 Sep 7. Biomaterials. 2007. PMID: 16959312
-
Dinuclear osmium(II) probes for high-resolution visualisation of cellular DNA structure using electron microscopy.Chem Commun (Camb). 2014 Dec 4;50(93):14494-7. doi: 10.1039/c4cc05547k. Chem Commun (Camb). 2014. PMID: 25198113
-
Fragmentation of fiberlike structures: sonication studies of cylindrical block copolymer micelles and behavioral comparisons to biological fibrils.J Am Chem Soc. 2008 Nov 5;130(44):14763-71. doi: 10.1021/ja805262v. Epub 2008 Oct 11. J Am Chem Soc. 2008. PMID: 18847270
Cited by
-
Enzyme-directed assembly and manipulation of organic nanomaterials.Chem Commun (Camb). 2011 Nov 21;47(43):11814-21. doi: 10.1039/c1cc15220c. Epub 2011 Sep 30. Chem Commun (Camb). 2011. PMID: 21959991 Free PMC article.
-
Functionalized DNA nanostructures as scaffolds for guided mineralization.Chem Sci. 2019 Sep 27;10(45):10537-10542. doi: 10.1039/c9sc02811k. eCollection 2019 Dec 7. Chem Sci. 2019. PMID: 32055376 Free PMC article.
-
Stimuli-responsive nanomaterials for biomedical applications.J Am Chem Soc. 2015 Feb 18;137(6):2140-54. doi: 10.1021/ja510147n. Epub 2015 Feb 6. J Am Chem Soc. 2015. PMID: 25474531 Free PMC article.
-
Precision spherical nucleic acids for delivery of anticancer drugs.Chem Sci. 2017 Sep 1;8(9):6218-6229. doi: 10.1039/c7sc01619k. Epub 2017 Jul 5. Chem Sci. 2017. PMID: 28989655 Free PMC article.
-
Spherical nucleic acids: Organized nucleotide aggregates as versatile nanomedicine.Aggregate (Hoboken). 2022 Feb;3(1):e120. doi: 10.1002/agt2.120. Epub 2021 Sep 14. Aggregate (Hoboken). 2022. PMID: 35386748 Free PMC article.
References
-
- Wang Y, Xu H, Zhang X. Adv Mater. 2009;21:2849. - PubMed
-
- Zhang L, Yu K, Eisenberg A. Science. 1996;272:1777. - PubMed
-
- Bütün V, Billingham NC, Armes SP. J Am Chem Soc. 1998;120:11818.
-
- LaRue I, Adam M, Pitsikalis M, Hadjichristidis N, Rubinstein M, Sheiko SS. Macromolecules. 2006;39:309.
-
- Liu X, Jiang M. Angew Chem. 2006;118:3930.
- Chem Int Ed. 2006;45:3846. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

