Effect of nanoparticle surface charge at the plasma membrane and beyond
- PMID: 20533851
- PMCID: PMC2925219
- DOI: 10.1021/nl101140t
Effect of nanoparticle surface charge at the plasma membrane and beyond
Abstract
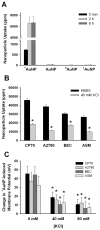
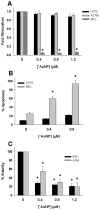
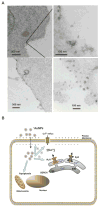
Herein, we demonstrate that the surface charge of gold nanoparticles (AuNPs) plays a critical role in modulating membrane potential of different malignant and nonmalignant cell types and subsequent downstream intracellular events. The findings presented here describe a novel mechanism for cell-nanoparticle interactions and AuNP uptake: modulation of membrane potential and its effect on intracellular events. These studies will help understand the biology of cell-nanoparticle interactions and facilitate the engineering of nanoparticles for specific intracellular targets.
Figures


Similar articles
-
Penetration of lipid membranes by gold nanoparticles: insights into cellular uptake, cytotoxicity, and their relationship.ACS Nano. 2010 Sep 28;4(9):5421-9. doi: 10.1021/nn1010792. ACS Nano. 2010. PMID: 20799717
-
Gold-Nanoparticle-Mediated Depolarization of Membrane Potential Is Dependent on Concentration and Tethering Distance from the Plasma Membrane.Bioconjug Chem. 2020 Mar 18;31(3):567-576. doi: 10.1021/acs.bioconjchem.9b00656. Epub 2020 Jan 14. Bioconjug Chem. 2020. PMID: 31894966
-
Gold nanoparticles with different charge and moiety induce differential cell response on mesenchymal stem cell osteogenesis.Biomaterials. 2015 Jun;54:226-36. doi: 10.1016/j.biomaterials.2015.03.001. Epub 2015 Apr 6. Biomaterials. 2015. PMID: 25858865
-
Gold Nanoparticles in Single-Cell Analysis for Surface Enhanced Raman Scattering.Molecules. 2016 Nov 25;21(12):1617. doi: 10.3390/molecules21121617. Molecules. 2016. PMID: 27897986 Free PMC article. Review.
-
Design and applications of gold nanoparticle conjugates by exploiting biomolecule-gold nanoparticle interactions.Nanoscale. 2013 Apr 7;5(7):2589-99. doi: 10.1039/c3nr33870c. Nanoscale. 2013. PMID: 23423633 Review.
Cited by
-
Development of a cationic polyethyleneimine-poly(lactic-co-glycolic acid) nanoparticle system for enhanced intracellular delivery of biologics.RSC Adv. 2023 Nov 17;13(48):33721-33735. doi: 10.1039/d3ra06050k. eCollection 2023 Nov 16. RSC Adv. 2023. PMID: 38020041 Free PMC article.
-
The membrane axis of Alzheimer's nanomedicine.Adv Nanobiomed Res. 2021 Jan;1(1):2000040. doi: 10.1002/anbr.202000040. Epub 2020 Nov 26. Adv Nanobiomed Res. 2021. PMID: 33748816 Free PMC article.
-
Biocompatible, Multi-Mode, Fluorescent, T2 MRI Contrast Magnetoelectric-Silica Nanoparticles (MagSiNs), for On-Demand Doxorubicin Delivery to Metastatic Cancer Cells.Pharmaceuticals (Basel). 2022 Sep 30;15(10):1216. doi: 10.3390/ph15101216. Pharmaceuticals (Basel). 2022. PMID: 36297329 Free PMC article.
-
Green pyomelanin-mediated synthesis of gold nanoparticles: modelling and design, physico-chemical and biological characteristics.Microb Cell Fact. 2019 Dec 3;18(1):210. doi: 10.1186/s12934-019-1254-2. Microb Cell Fact. 2019. PMID: 31796078 Free PMC article.
-
Role of Surface Hydrophobicity of Dicationic Amphiphile-Stabilized Gold Nanoparticles on A549 Lung Cancer Cells.ACS Omega. 2017 Jul 31;2(7):3527-3538. doi: 10.1021/acsomega.7b00353. Epub 2017 Jul 25. ACS Omega. 2017. PMID: 30023697 Free PMC article.
References
-
- Ferrari M. Nat Rev Cancer. 2005;5(3):161–71. - PubMed
-
- Burda C, Chen X, Narayanan R, El-Sayed MA. Chem Rev. 2005;105(4):1025–102. - PubMed
-
- Daniel MC, Astruc D. Chem Rev. 2004;104(1):293–346. - PubMed
-
- Patra CR, Bhattacharya R, Wang E, Katarya A, Lau JS, Dutta S, Muders M, Wang S, Buhrow SA, Safgren SL, Yaszemski MJ, Reid JM, Ames MM, Mukherjee P, Mukhopadhyay D. Cancer Res. 2008;68(6):1970–8. - PubMed
-
- Mirkin CA, Taton TA. Nature. 2000;405(6787):626–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

