Physical mechanisms of signal integration by WASP family proteins
- PMID: 20533885
- PMCID: PMC3017724
- DOI: 10.1146/annurev.biochem.77.060407.135452
Physical mechanisms of signal integration by WASP family proteins
Abstract
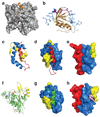
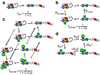
The proteins of the Wiskott-Aldrich syndrome protein (WASP) family are activators of the ubiquitous actin nucleation factor, the Arp2/3 complex. WASP family proteins contain a C-terminal VCA domain that binds and activates the Arp2/3 complex in response to numerous inputs, including Rho family GTPases, phosphoinositide lipids, SH3 domain-containing proteins, kinases, and phosphatases. In the archetypal members of the family, WASP and N-WASP, these signals are integrated through two levels of regulation, an allosteric autoinhibitory interaction, in which the VCA is sequestered from the Arp2/3 complex, and dimerization/oligomerization, in which multi-VCA complexes are better activators of the Arp2/3 complex than monomers. Here, we review the structural, biochemical, and biophysical details of these mechanisms and illustrate how they work together to control WASP activity in response to multiple inputs. These regulatory principles, derived from studies of WASP and N-WASP, are likely to apply broadly across the family.
Figures



References
-
- Pollard TD. Regulation of actin filament assembly by Arp2/3 complex and formins. Annu Rev Biophys Biomol Struct. 2007;36:451–477. - PubMed
-
- Chhabra ES, Higgs HN. The many faces of actin: matching assembly factors with cellular structures. Nat Cell Biol. 2007;9:1110–1121. - PubMed
-
- Stradal TE, Scita G. Protein complexes regulating Arp2/3-mediated actin assembly. Curr Opin Cell Biol. 2006;18:4–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

