Mitochondrial fission and fusion
- PMID: 20533902
- PMCID: PMC4762097
- DOI: 10.1042/bse0470085
Mitochondrial fission and fusion
Abstract
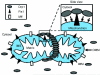
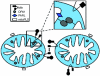
Mitochondria are highly dynamic cellular organelles, with the ability to change size, shape and position over the course of a few seconds. Many of these changes are related to the ability of mitochondria to undergo the highly co-ordinated processes of fission (division of a single organelle into two or more independent structures) or fusion (the opposing reaction). These actions occur simultaneously and continuously in many cell types, and the balance between them regulates the overall morphology of mitochondria within any given cell. Fission and fusion are active processes which require many specialized proteins, including mechanical enzymes that physically alter mitochondrial membranes, and adaptor proteins that regulate the interaction of these mechanical proteins with organelles. Although not fully understood, alterations in mitochondrial morphology appear to be involved in several activities that are crucial to the health of cells. In the present chapter we discuss the mechanisms behind mitochondrial fission and fusion, and discuss the implications of changes in organelle morphology during the life of a cell.
Figures

References
-
- Kennedy EP, Lehninger AL. Oxidation of fatty acids and tricarboxylic acid cycle intermediates by isolated rat liver mitochondria. J. Biol. Chem. 1949;179:957–972. - PubMed
-
- Lewis MR, Lewis WH. Mitochondria in tissue cultures. Science. 1914;39:330–333. - PubMed
-
- Zimmer C. On the origin of eukaryotes. Science. 2009;325:666–668. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

